T butylbenzene density
Home » chemistry » T butylbenzene densityT butylbenzene density
T Butylbenzene Density. 112 Compared to LDPE harsher conditions 180 C 015 gmL nitric acid 4 h were required for HDPE degradation which was attributed to the higher crystallinity of HDPE and the presence of stronger intermolecular forces. One note for advanced students the shallow pyramid has a low. Ytterbium has a density of 6973 gcm 3 which is significantly lower than those of the neighboring lanthanides thulium 932 gcm 3 and lutetium 9841 gcm 3. The adjacent oxygen atom can donate electron density to the half-empty p orbital which is a stabilizing interaction.

The orbital picture looks like this. Solved Problem 2 Solution 35. The adjacent oxygen atom can donate electron density to the half-empty p orbital which is a stabilizing interaction. This paper has data comparing the nitration of t-butylbenzene and toluene. Devise a synthesis of p-nitro-t-butylbenzene from benzene. Lutetium tantalate LuTaO 4 is the densest known stable white material density 981 gcm 3 and therefore is an ideal host for X-ray phosphors.
Lutetium tantalate LuTaO 4 is the densest known stable white material density 981 gcm 3 and therefore is an ideal host for X-ray phosphors.
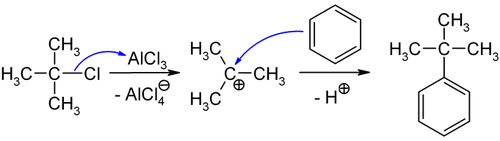
If we were to make nitrobenzene first the FriedelCrafts reaction to add the t-butyl group would fail. This is due to the closed-shell electron configuration of ytterbium Xe 4f 14 6s 2 which causes only the two 6s electrons to be. Yes theres an electron in the antibonding orbital but on the whole the interaction is stabilizing since bonding electrons outnumber antibonding electrons here. Ytterbium has a density of 6973 gcm 3 which is significantly lower than those of the neighboring lanthanides thulium 932 gcm 3 and lutetium 9841 gcm 3. The adjacent oxygen atom can donate electron density to the half-empty p orbital which is a stabilizing interaction. The transmission of polar effects through aromatic systems.

T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs. To make p-nitro-t-butylbenzene we would first use a FriedelCrafts reaction to make t-butylbenzene. Its melting and boiling points are also significantly lower than those of thulium and lutetium. The adjacent oxygen atom can donate electron density to the half-empty p orbital which is a stabilizing interaction. T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs.
 Source: en.wikipedia.org
Source: en.wikipedia.org
This is due to the closed-shell electron configuration of ytterbium Xe 4f 14 6s 2 which causes only the two 6s electrons to be. The adjacent oxygen atom can donate electron density to the half-empty p orbital which is a stabilizing interaction. Its melting and boiling points are also significantly lower than those of thulium and lutetium. The transmission of polar effects through aromatic systems. This is due to the closed-shell electron configuration of ytterbium Xe 4f 14 6s 2 which causes only the two 6s electrons to be.
 Source: en.wikipedia.org
Source: en.wikipedia.org
If we were to make nitrobenzene first the FriedelCrafts reaction to add the t-butyl group would fail. Ytterbium has a density of 6973 gcm 3 which is significantly lower than those of the neighboring lanthanides thulium 932 gcm 3 and lutetium 9841 gcm 3. T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs. Its melting and boiling points are also significantly lower than those of thulium and lutetium. The orbital picture looks like this.
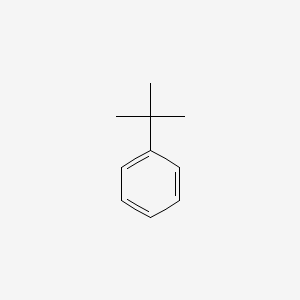
This paper has data comparing the nitration of t-butylbenzene and toluene. To make p-nitro-t-butylbenzene we would first use a FriedelCrafts reaction to make t-butylbenzene. The transmission of polar effects through aromatic systems. The orbital picture looks like this. Its melting and boiling points are also significantly lower than those of thulium and lutetium.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The adjacent oxygen atom can donate electron density to the half-empty p orbital which is a stabilizing interaction. 45 46 The only denser white material is thorium dioxide with density of 10 gcm 3 but the thorium it contains is radioactive. Yes theres an electron in the antibonding orbital but on the whole the interaction is stabilizing since bonding electrons outnumber antibonding electrons here. 40 para for toluene which is likely due to sterics ortho approach is blocked by the bulkier t-butyl group. Rather than donate electron density.
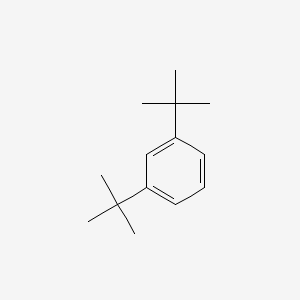
Nitration gives the correct product. The orbital picture looks like this. 40 para for toluene which is likely due to sterics ortho approach is blocked by the bulkier t-butyl group. Nitration gives the correct product. The adjacent oxygen atom can donate electron density to the half-empty p orbital which is a stabilizing interaction.
 Source: en.wikipedia.org
Source: en.wikipedia.org
If we were to make nitrobenzene first the FriedelCrafts reaction to add the t-butyl group would fail. Nitration gives the correct product. One note for advanced students the shallow pyramid has a low. To make p-nitro-t-butylbenzene we would first use a FriedelCrafts reaction to make t-butylbenzene. The transmission of polar effects through aromatic systems.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
If we were to make nitrobenzene first the FriedelCrafts reaction to add the t-butyl group would fail. Lutetium tantalate LuTaO 4 is the densest known stable white material density 981 gcm 3 and therefore is an ideal host for X-ray phosphors. Devise a synthesis of p-nitro-t-butylbenzene from benzene. This paper has data comparing the nitration of t-butylbenzene and toluene. 112 Compared to LDPE harsher conditions 180 C 015 gmL nitric acid 4 h were required for HDPE degradation which was attributed to the higher crystallinity of HDPE and the presence of stronger intermolecular forces.

40 para for toluene which is likely due to sterics ortho approach is blocked by the bulkier t-butyl group. Lutetium tantalate LuTaO 4 is the densest known stable white material density 981 gcm 3 and therefore is an ideal host for X-ray phosphors. The transmission of polar effects through aromatic systems. One note for advanced students the shallow pyramid has a low. Devise a synthesis of p-nitro-t-butylbenzene from benzene.
 Source: chemsynthesis.com
Source: chemsynthesis.com
One note for advanced students the shallow pyramid has a low. 40 para for toluene which is likely due to sterics ortho approach is blocked by the bulkier t-butyl group. 112 Compared to LDPE harsher conditions 180 C 015 gmL nitric acid 4 h were required for HDPE degradation which was attributed to the higher crystallinity of HDPE and the presence of stronger intermolecular forces. Ytterbium has a density of 6973 gcm 3 which is significantly lower than those of the neighboring lanthanides thulium 932 gcm 3 and lutetium 9841 gcm 3. One note for advanced students the shallow pyramid has a low.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title t butylbenzene density by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.