Sodium iodide density
Home » chemistry » Sodium iodide densitySodium iodide density
Sodium Iodide Density. Sodium iodate comprises 15 to 50 mg per kilogram of applicable salt. However existing solid ion conductors are limited in their capacity to stand up to the rigors of battery operations. Sodium hydroxide NaOH or HNaO CID 14798 - structure chemical names physical and chemical properties classification patents literature biological activities. Radiolabelled compound DB09293 is used as a diagnostic tool to evaluate thyroid function and morphology.

Sodium is the sixth most abundant. Sodium is an alkali metal being in group 1 of the periodic table. What is the weightvolume percentage concentration of 250 mL of aqueous sodium chloride solution containing 5 g NaCl. Density near rt 0968 gcm 3. Step 1 Write the equation. Step 2 Identify the solute and solvent by name or chemical formula solute sodium chloride NaCl.
Density near rt 0968 gcm 3.
Sodium or its compounds can turn a flame yellow and can burn in the air with a brilliant yellow flame. It is a soft silvery-white highly reactive metal. Solvent is water H 2 O because this is an aqueous. Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11. Sodium has extremely low density and it is a bit lower than that of water. This is our newest publication and has been created to support the school technician profession in Scotland.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine. Sodium iodate is also used as a dough conditioner to strengthen the dough. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11. Sodium is the sixth most abundant.
 Source: en.wikipedia.org
Source: en.wikipedia.org
For this reason if you place it in water it will float. Because the bulk density of a substance varies greatly depending on how the material has been handled the information contained in this reference tool should be used for estimation purposes only. It is highly anticipated that next-generation solid-state batteries with lithium-metal anodes will usher in a new wave of energy storage systems that are both safer than conventional lithium-ion li-ion battery cells and deliver higher energy densities. Solvent is water H 2 O because this is an aqueous. Though after some time it will react violently on contact with water and release large amounts of heat that may result in outbreak of flames.
 Source: scbt.com
Source: scbt.com
Sodium hydroxide NaOH or HNaO CID 14798 - structure chemical names physical and chemical properties classification patents literature biological activities. It is a soft silvery-white highly reactive metal. Sodium hydroxide NaOH or HNaO CID 14798 - structure chemical names physical and chemical properties classification patents literature biological activities. Sodium has extremely low density and it is a bit lower than that of water. For this reason if you place it in water it will float.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
However existing solid ion conductors are limited in their capacity to stand up to the rigors of battery operations. Sodium iodate is also used as a dough conditioner to strengthen the dough. Sodium is the sixth most abundant. Solvent is water H 2 O because this is an aqueous. Sodium iodate comprises 15 to 50 mg per kilogram of applicable salt.

However existing solid ion conductors are limited in their capacity to stand up to the rigors of battery operations. For this reason if you place it in water it will float. Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine. Sodium or its compounds can turn a flame yellow and can burn in the air with a brilliant yellow flame. Step 1 Write the equation.
 Source: sciencedirect.com
Source: sciencedirect.com
Step 1 Write the equation. Density near rt 0968 gcm 3. Its only stable isotope is 23 Na. Because the bulk density of a substance varies greatly depending on how the material has been handled the information contained in this reference tool should be used for estimation purposes only. Sodium is the sixth most abundant.

Step 2 Identify the solute and solvent by name or chemical formula solute sodium chloride NaCl. Sodium hydroxide NaOH or HNaO CID 14798 - structure chemical names physical and chemical properties classification patents literature biological activities. What is the weightvolume percentage concentration of 250 mL of aqueous sodium chloride solution containing 5 g NaCl. Because the bulk density of a substance varies greatly depending on how the material has been handled the information contained in this reference tool should be used for estimation purposes only. Step 1 Write the equation.
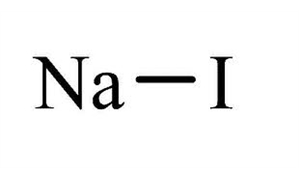 Source: lobachemie.com
Source: lobachemie.com
Sodium has extremely low density and it is a bit lower than that of water. Because the bulk density of a substance varies greatly depending on how the material has been handled the information contained in this reference tool should be used for estimation purposes only. Sodium has extremely low density and it is a bit lower than that of water. You can scroll through the entire list or start typing the name of a compound in the search field all materials containing those characters will be displayed. The main use of sodium iodate in everyday life is in iodised salt.
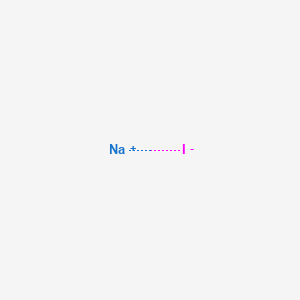
For this reason if you place it in water it will float. Sodium is an alkali metal being in group 1 of the periodic table. This is our newest publication and has been created to support the school technician profession in Scotland. Its only stable isotope is 23 Na. Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine.
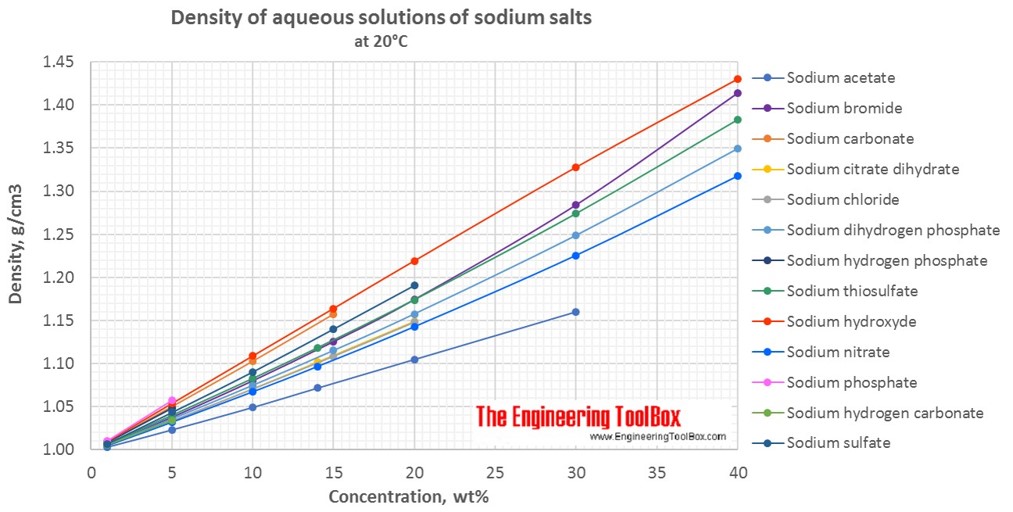 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Sodium is the sixth most abundant. Though after some time it will react violently on contact with water and release large amounts of heat that may result in outbreak of flames. Sodium iodate is also used as a dough conditioner to strengthen the dough. What is the weightvolume percentage concentration of 250 mL of aqueous sodium chloride solution containing 5 g NaCl.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium iodide density by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.