Nitrogen dioxide density
Home » chemistry » Nitrogen dioxide densityNitrogen dioxide density
Nitrogen Dioxide Density. The calculator below can be used to estimate the density and specific weight of gaseous nitrogen at given temperature and pressure. Carl Wilhelm Scheele a Swedish chemist showed in 1772 that air is a mixture of two gases one of which he. The output density is given as kgm 3 lbft 3 lbgalUS liq and. Nitrogen dioxide absorption and sulfite oxidation in aqueous sulfite.
 Nitrogen Dioxide Wikipedia From en.wikipedia.org
Nitrogen Dioxide Wikipedia From en.wikipedia.org
26 Facts about Nitrogen. Ground-level ozone is formed by photochemical reactions between sunlight and pollutant precursors such as nitrogen oxides and volatile organic compounds especially in warm conditions and peaks in summer temperatures. When heated under pressure with hydrogen. Over 98 percent of man made N0 emissions result from Combustion with the majority due to stationary sources. As a liquid it is also colourless and odourless. A particle density of 15 g cm 3.
The key to most efficiently using urea is to incorporate it into the soil during a tillage operation.
Melting Point of Nitrogen dioxide. Density of Nitrogen dioxide. The Gas Cost Estimator provides a budgetary cost estimate and sample system layout and sketch for bulk argon nitrogen or oxygen supply after you provide some basic information regarding your requirements location volume usage etc. The word nitrogen therefore means nitre former. During the quarantine roads and transportation hubs are emptier. It is one of several nitrogen oxides.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It can be fatal if inhaled in large. Ground-level ozone is formed by photochemical reactions between sunlight and pollutant precursors such as nitrogen oxides and volatile organic compounds especially in warm conditions and peaks in summer temperatures. 26 Facts about Nitrogen. As the oxygen in the air is pushed out of the beaker by the denser carbon dioxide the candles go out one by one. NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizers.
 Source: researchgate.net
Source: researchgate.net
Combustion generated oxides of nitrogen are emitted predominantly as nitric oxide N0 a relatively harmless gas. Synonyms Trade Names Dinitrogen tetroxide Nitrogen peroxide CAS No. DOT ID Guide. Bacteria transform airborne nitrogen and carbon dioxide into functional components that can be used as basic building blocks by plants and animals. 1s 2 2s 2 2p 3.
 Source: researchgate.net
Source: researchgate.net
For more information on NASAs long-term measurements of nitrogen dioxide please see this page. Boiling Point of Nitrogen dioxide. For more information on NASAs long-term measurements of nitrogen dioxide please see this page. Ground-level ozone is formed by photochemical reactions between sunlight and pollutant precursors such as nitrogen oxides and volatile organic compounds especially in warm conditions and peaks in summer temperatures. Over 98 percent of man made N0 emissions result from Combustion with the majority due to stationary sources.
 Source: researchgate.net
Source: researchgate.net
For more information on NASAs long-term measurements of nitrogen dioxide please see this page. In this state the energy density of ammonia is about 3 kWhlitre which is less than but. 66 Nitrogen oxides including nitrogen dioxide are formed primarily by the reaction of ozone with nitric oxide emitted during fossil fuel combustion. With these facts about nitrogen let us learn about its chemistry physical properties atomic mass and much more. An example is nitrate derived from artificial fertilizer manure power-plant emissions or.
 Source: researchgate.net
Source: researchgate.net
The compound used to. NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizers. Nitrogen is an inert gas with many industrial applications. Nitrogen from urea can be lost to the atmosphere if fertilizer urea remains on the soil surface for extended periods of time during warm weather. Density g cm3 0001145.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
Boiling Point of Nitrogen dioxide. To store in bulk it requires liquefaction either by compression to 10 times atmospheric pressure or chilling to -33C. When mixed with oxygen and subjected to electric sparks it forms nitric oxide NO and then the dioxide NO 2. Methane produces carbon dioxide whereas the nitrogen atom in ammonia results in nitrogen gas N 2. Ground-level ozone is formed by photochemical reactions between sunlight and pollutant precursors such as nitrogen oxides and volatile organic compounds especially in warm conditions and peaks in summer temperatures.
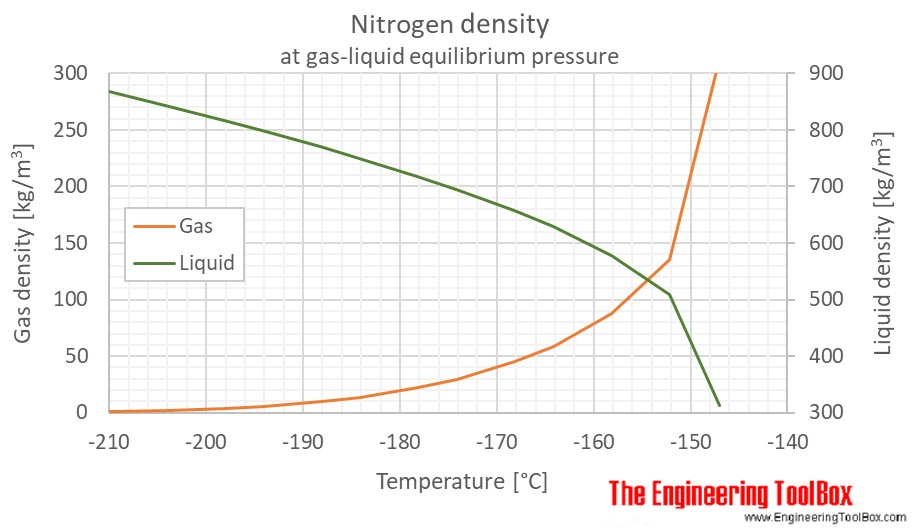 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
3 3 5. It is one of several nitrogen oxides. As a liquid it is also colourless and odourless. The calculator below can be used to estimate the density and specific weight of gaseous nitrogen at given temperature and pressure. 26 Facts about Nitrogen.
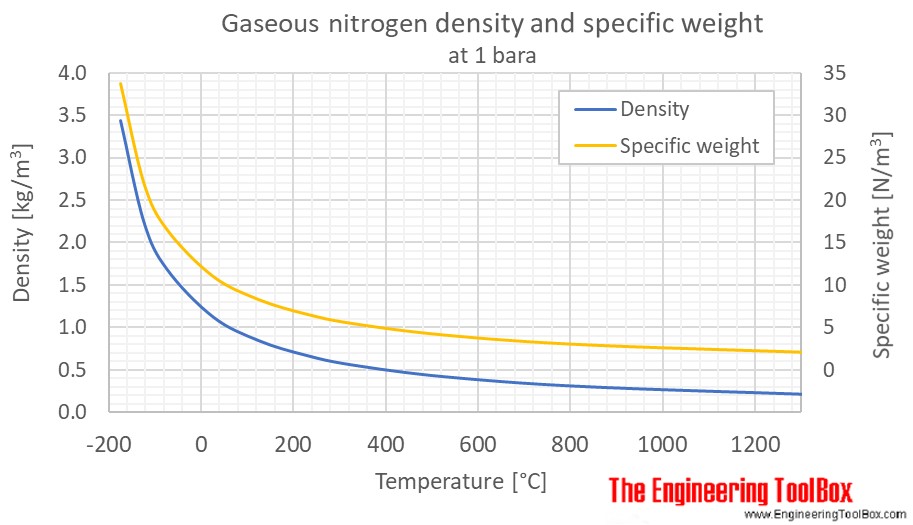 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It is one of several nitrogen oxides. When nitrogen is heated it combines directly with magnesium lithium or calcium. In the process the air which is less dense is pushed up and out of the beaker. 26 Facts about Nitrogen. Nitrous oxide N2O is a greenhouse gas with significant anthropogenic sources contributing to its worldwide abundance 03 ppm.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Atomic nitrogen is prepared by passing an electric discharge through. Boiling Point of Nitrogen dioxide. Over 98 percent of man made N0 emissions result from Combustion with the majority due to stationary sources. 66 Nitrogen oxides including nitrogen dioxide are formed primarily by the reaction of ozone with nitric oxide emitted during fossil fuel combustion. Variations in the isotope-amount ratio n15 Nn14 N are used to determine sources of nitrogen contamination in the atmosphere oceans groundwater and rivers where the isotopic composition of a contaminant molecule preserves evidence of the nitrogen sources and processes involved in its creation.
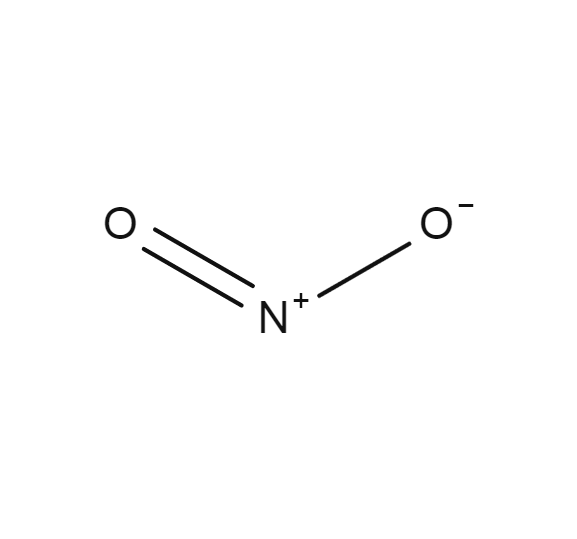 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Atomic nitrogen is prepared by passing an electric discharge through. Density 1 atm 0 C 12506 gramslitre. The word nitrogen therefore means nitre former. 3 3 5. In this case the carbon dioxide produced by the vinegar and baking soda reaction sinks to the bottom of the beaker.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title nitrogen dioxide density by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.