Lithium chloride density
Home » chemistry » Lithium chloride densityLithium chloride density
Lithium Chloride Density. Although lithium compounds are known to stabilize mood scientists. On the other hand ice solid H 2 O is a molecular compound whose molecules are held together by hydrogen bonds which is effectively a strong example of an interaction between two permanent dipoles. 12 3480634814 2020. Lithium-sulphur batteries LSBs have one of the great potentials for serving as next generation high energy density batteries.
 Msds For Lithium Chloride From studylib.net
Msds For Lithium Chloride From studylib.net
Caution is advised in patients treated with lithium. This is in part because lithium is the third-smallest element after hydrogen and helium and thus a lithium ion can carry a positive charge in a very small amount of space. Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. Administration of Sodium Chloride 09 may result in decreased lithium levels. Properties of Lithium. It has the highest specific heat of any.
Under the broad category of primary lithium battery types numerous types of primary lithium battery chemistries are commercially-available that differ in their performance characteristics.
See Section 44 Special warnings and. Renal sodium and lithium clearance may be increased during administration of Sodium Chloride 09. Lithium chloride is one of the most hygroscopic materials known and it as well as lithium bromide is used in air conditioning and industrial drying systems. Nearly all of the US. Acetic acid acetic aldehyde acetone. This is in part because lithium is the third-smallest element after hydrogen and helium and thus a lithium ion can carry a positive charge in a very small amount of space.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Other lithium compounds are used in dry cells and storage batteries. Properties of Lithium. Lithium metal is produced through electrolysis from a mixture of fused 55 lithium chloride and 45 potassium chloride at about 450 C. Alloys of the metal with manganese cadmium copper and aluminium are used to make aircrafts parts. As per recent market research reports the graphene battery market including graphene battery lithium-sulphur battery and graphene supercapacitors end use industries like electronics automotive power etc the market size is projected to grow from 168 million USD.
 Source: studylib.net
Source: studylib.net
Sodium chloride melts at 801C. Depending on the design and chemical compounds used lithium cells can produce voltages from 15 V comparable to a zinccarbon or alkaline battery. Lithium country mine production 2006 metric tons of world known mine production. Caution is advised in patients treated with lithium. CorticoidsSteroids and carbenoxolone are associated with the retention of sodium and water with oedema and hypertension.
 Source: researchgate.net
Source: researchgate.net
Properties of Lithium. Brine is used in the manufacturing of air conditioning systems. Lithium stearate is used as an all-purpose and high-temperature lubricant. Depending on the design and chemical compounds used lithium cells can produce voltages from 15 V comparable to a zinccarbon or alkaline battery. CorticoidsSteroids and carbenoxolone are associated with the retention of sodium and water with oedema and hypertension.
 Source: researchgate.net
Source: researchgate.net
CorticoidsSteroids and carbenoxolone are associated with the retention of sodium and water with oedema and hypertension. Other lithium compounds are used in dry cells and storage batteries. Although lithium compounds are known to stabilize mood scientists. Lithium-ion batteries can be. On the other hand ice solid H 2 O is a molecular compound whose molecules are held together by hydrogen bonds which is effectively a strong example of an interaction between two permanent dipoles.
 Source: sciencedirect.com
Source: sciencedirect.com
Lithium metal is produced through electrolysis from a mixture of fused 55 lithium chloride and 45 potassium chloride at about 450 C. Used in batteries lithium provides much better energy per volume ratio or energy density than an ordinary alkaline battery or other common rechargeable battery such as a nickel-metal hydride. Like lithium carbonate and lithium chloride it was used as treatment for bipolar disorder. Lithium stock is currently imported. Though hydrogen bonds are the strongest of the intermolecular forces the strength of hydrogen bonds is much less than that of ionic.
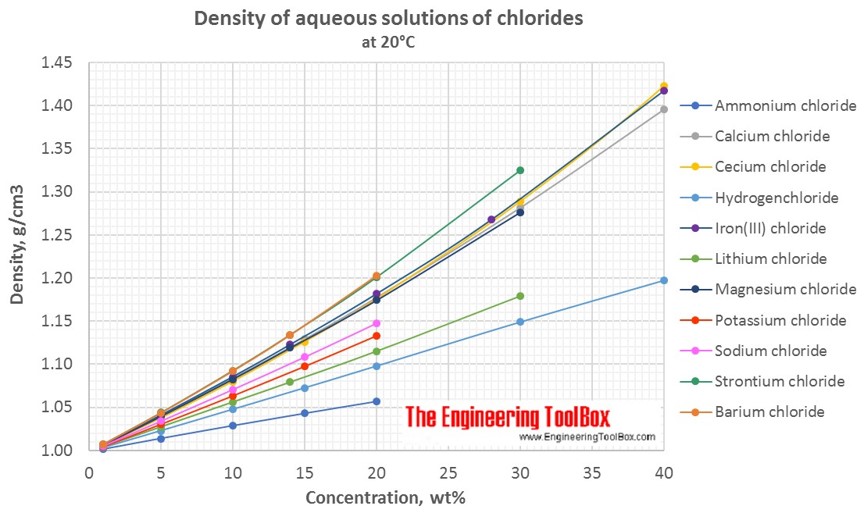 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
In other words if lithium didnt react with water which it does somewhat vigorously it would float. Theoretical design of lithium chloride superionic conductors for all-solid-state high-voltage lithium-ion batteries. Bromine and lithium chloride together form concentrated brine which absorbs the humidity under high temperature. CorticoidsSteroids and carbenoxolone are associated with the retention of sodium and water with oedema and hypertension. Brine is used in the manufacturing of air conditioning systems.
 Source: studylib.net
Source: studylib.net
It is the lightest of the metals with a density approximately half that of water. See Section 44 Special warnings and. Administration of Sodium Chloride 09 may result in decreased lithium levels. This is in part because lithium is the third-smallest element after hydrogen and helium and thus a lithium ion can carry a positive charge in a very small amount of space. As domestic demand increases rapidly there is an urgent need to develop safe secure domestic and cost-competitive sources of lithium.
 Source: studylib.net
Source: studylib.net
Caution is advised in patients treated with lithium. Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. Lithium Thionyl Chloride Li-SOCl₂ chemistry is commonly utilised in industrial remote and medical applications. Under ordinary conditions lithium is the least dense of the solid elements. As domestic demand increases rapidly there is an urgent need to develop safe secure domestic and cost-competitive sources of lithium.
 Source: studylib.net
Source: studylib.net
They stand apart from other batteries in their high charge density and high cost per unit. Lithium Properties. It has the highest specific heat of any. Due to the growth in EV technology as well as concerns over increased CO2 pollution from combustion engines and rising fuel costs lithium has been put into widespread use in EV batteries. Properties of Lithium.
 Source: researchgate.net
Source: researchgate.net
On the other hand ice solid H 2 O is a molecular compound whose molecules are held together by hydrogen bonds which is effectively a strong example of an interaction between two permanent dipoles. The table lists the major producers of lithium. Depending on the design and chemical compounds used lithium cells can produce voltages from 15 V comparable to a zinccarbon or alkaline battery. Though hydrogen bonds are the strongest of the intermolecular forces the strength of hydrogen bonds is much less than that of ionic. Lithium chloride is one of the most hygroscopic materials known and is used in air conditioning and industrial drying systems as is lithium bromide.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lithium chloride density by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.