Hcl density hazards
Home » chemistry » Hcl density hazardsHcl density hazards
Hcl Density Hazards. Consequently the molecule has a large dipole moment with a negative partial charge δ at the chlorine atom and a positive partial charge δ at the hydrogen. Where the neat test chemical is weighed and diluted wear a NIOSH-approved half face respirator equipped with an organic vaporacid gas cartridge specific for organic vapors HCl acid gas and SO2 with a dustmist filter. CAS 7647-01-0 is listed as a hazardous air pollutant HAP. Unfortunately however our sense of smell is not a reliable alarm - at mixing.
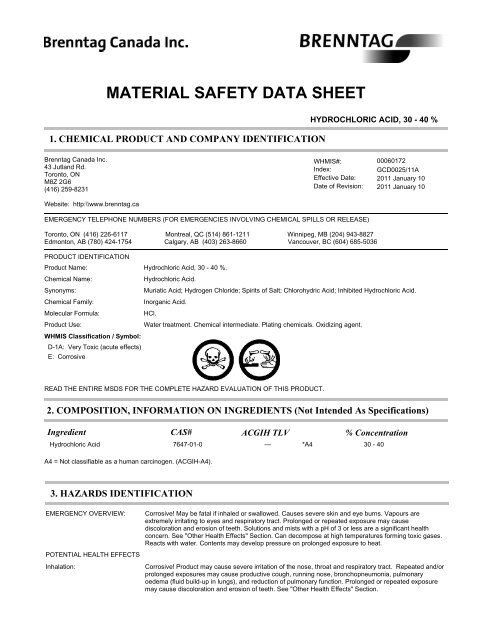 Hydrochloric Acid Msds English Pdf Bartlett Ca From yumpu.com
Hydrochloric Acid Msds English Pdf Bartlett Ca From yumpu.com
The 1988 PTWI was not reconsidered and was maintained at the fifty-fifth meeting 2000. This material contains Hydrogen chloride CAS 7647-01-0 32-38which is subject to the reporting requirements of Section 313 of SARA Title III and 40 CFR Part 373. Before performing this experiment ie in your prelab calculate the volume of concentrated HCl you will need to prepare 250 mL of 01M HCl. 069 gmL Boiling point. 326 K Hazards Safety data sheet. This material does not contain any Class 1 Ozone depletors.
Air mixtures with as little as 0000001 H 2 S are associated with a rotten egg smell.
Put 100 mL distilled water into your other large bottle. Appearance colorless liquid Density. 069 gmL Boiling point. 326 K Hazards Safety data sheet. Air mixtures with as little as 0000001 H 2 S are associated with a rotten egg smell. CAS 7647-01-0 is listed as a hazardous air pollutant HAP.
 Source: yumpu.com
Source: yumpu.com
069 gmL Boiling point. 98220 gmol 1. 53 C 127 F. Concentrated reagent grade HCl has a density of 1188 g mL1 and is 37 wt. The Committee reiterated the conclusion reached in 1988 that tin concentrations as low as 150 mgkg in canned beverages.
 Source: studylib.net
Source: studylib.net
PTWI expressed as Sn including stannous chloride. Preparation of Approximate Acid Solution 01M HCl 1. Air mixtures with as little as 0000001 H 2 S are associated with a rotten egg smell. PTWI expressed as Sn including stannous chloride. Hydrogen sulfide is a colorless flammable gas with a strong offensive odor.
 Source: yumpu.com
Source: yumpu.com
PTWI expressed as Sn including stannous chloride. Reacts exothermically with carbonates including limestone and building materials containing limestone and hydrogen carbonates to generate carbon dioxide. Before performing this experiment ie in your prelab calculate the volume of concentrated HCl you will need to prepare 250 mL of 01M HCl. 53 C 127 F. Hydrogen sulfide is a colorless flammable gas with a strong offensive odor.
 Source: studylib.net
Source: studylib.net
The Committee reiterated the conclusion reached in 1988 that tin concentrations as low as 150 mgkg in canned beverages. The Committee reiterated the conclusion reached in 1988 that tin concentrations as low as 150 mgkg in canned beverages. 53 C 127 F. The 1988 PTWI was not reconsidered and was maintained at the fifty-fifth meeting 2000. Air mixtures with as little as 0000001 H 2 S are associated with a rotten egg smell.
 Source: simplebooklet.com
Source: simplebooklet.com
Reacts with sulfides carbides borides and phosphides to generate toxic. This material does not contain any Class 1 Ozone depletors. Interestingly the human nose is more sensitive to H 2 S than any gas monitoring instrument we have today. 069 gmL Boiling point. Consequently the molecule has a large dipole moment with a negative partial charge δ at the chlorine atom and a positive partial charge δ at the hydrogen.
 Source: yumpu.com
Source: yumpu.com
C 5 H 10 Si. This material contains Hydrogen chloride CAS 7647-01-0 32-38which is subject to the reporting requirements of Section 313 of SARA Title III and 40 CFR Part 373. 98220 gmol 1. ANHYDROUS HYDROGEN CHLORIDE is an anhydrous no water strong acid. The 1988 PTWI was not reconsidered and was maintained at the fifty-fifth meeting 2000.
 Source: www2.atmos.umd.edu
Source: www2.atmos.umd.edu
Where the neat test chemical is weighed and diluted wear a NIOSH-approved half face respirator equipped with an organic vaporacid gas cartridge specific for organic vapors HCl acid gas and SO2 with a dustmist filter. Hydrogen sulfide is a colorless flammable gas with a strong offensive odor. The 1988 PTWI was not reconsidered and was maintained at the fifty-fifth meeting 2000. Unfortunately however our sense of smell is not a reliable alarm - at mixing. Reacts rapidly and exothermically with bases of all kinds including amines and amides.

326 K Hazards Safety data sheet. It is sometimes referred to as sewer gas. This material contains Hydrogen chloride CAS 7647-01-0 32-38which is subject to the reporting requirements of Section 313 of SARA Title III and 40 CFR Part 373. PTWI expressed as Sn including stannous chloride. Splash proof safety goggles should be worn while handling this chemical.
 Source: slideserve.com
Source: slideserve.com
This material does not contain any Class 1 Ozone depletors. PTWI expressed as Sn including stannous chloride. ANHYDROUS HYDROGEN CHLORIDE is an anhydrous no water strong acid. Unfortunately however our sense of smell is not a reliable alarm - at mixing. Where the neat test chemical is weighed and diluted wear a NIOSH-approved half face respirator equipped with an organic vaporacid gas cartridge specific for organic vapors HCl acid gas and SO2 with a dustmist filter.
 Source: studylib.net
Source: studylib.net
326 K Hazards Safety data sheet. Hydrogen sulfide is a colorless flammable gas with a strong offensive odor. Before performing this experiment ie in your prelab calculate the volume of concentrated HCl you will need to prepare 250 mL of 01M HCl. Reacts exothermically with carbonates including limestone and building materials containing limestone and hydrogen carbonates to generate carbon dioxide. Concentrated reagent grade HCl has a density of 1188 g mL1 and is 37 wt.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hcl density hazards by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.