Given that the density of acetic anhydride is 108
Home » chemistry » Given that the density of acetic anhydride is 108Given that the density of acetic anhydride is 108
Given That The Density Of Acetic Anhydride Is 108. A decent rule of thumb is that you will never ever see a CO stretch below 1630. Academiaedu is a platform for academics to share research papers. In addition the density of water is 10 gmL or 10 mg0001 mL which makes the conversion between the two units easier. Acetic Anhydride Den 108 gml.
 Solved Given The Density Of Acetic Anhydride 1 08 G Ml Chegg Com From chegg.com
Solved Given The Density Of Acetic Anhydride 1 08 G Ml Chegg Com From chegg.com
164 HBr has. Academiaedu is a platform for academics to share research papers. Where H is the distance between the tip of the spinneret and the collector h is the length of the spinneret and R is the outer radius of the spinneret. This dissolved material will be given to an IA who will run your sample on the HPLC and give you a copy of the chromatogram of your crude aspirin. It is something else. A 747 jet is filled with 173000 L of jet fuel.
For example if we find that there is lead contamination in water of 4 ppm this would mean that there are.
The IUPAC name of the compound having the formula CH32CHCH2CH2Br is A. Take about 2 grams of your slightly moist aspirin isolated above and add to a small beaker. Theweightof air exhausted will however be di- rectly proportional to the density and so will the static pressure developed and the horsepower consumed. M1 and M2 are the molecular weights of component 1 and 2 respectively. The remaining water if any exists. Given C-C single bond length is 154 A.
 Source: numerade.com
Source: numerade.com
The IUPAC name of the compound having the formula CH32CHCH2CH2Br is A. The IUPAC name of the compound having the formula CH32CHCH2CH2Br is A. The average molar mass of chlorine is 355 g mol1. Pure acetic acid known as glacial acetic acid is a liquid with a density of 1049 gmL at 25OC. It is something else.
 Source: bartleby.com
Source: bartleby.com
The remaining water if any exists. Where H is the distance between the tip of the spinneret and the collector h is the length of the spinneret and R is the outer radius of the spinneret. Add either 5 mL of methanol or 5. Calculate the molarity of a solution of acetic acid made by dissolving 100 mL of glacial acetic acid at 25OC in enough water to make 100 mL of solution. The average molar mass of chlorine is 355 g mol1.
 Source: study.com
Source: study.com
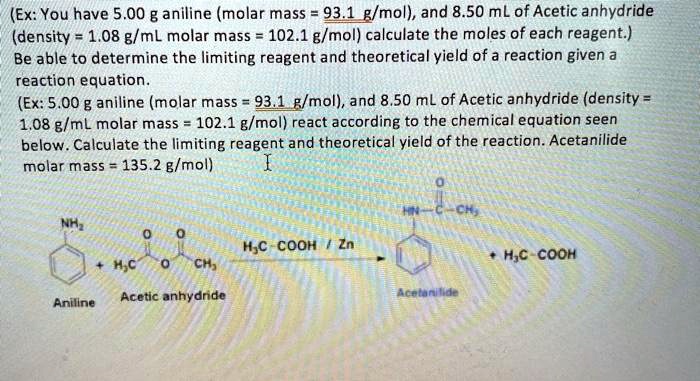
BRILLIANT PUBLIC SCHOOL SITAMARHI Affiliated up to 2 level to CBSE New Delhi Class-XI IIT-JEE Advanced Chemistry Study Package Session. The density of acetic anhydride is 108 gmL. Density 108 gmL View Answer. Compute actual molar ratio of reactants 3. The balanced equation is.
 Source: chegg.com
Source: chegg.com
Once the reaction is complete the. Acetic Anhydride Den 108 gml. Given C-C single bond length is 154 A. Density 108 gmL View Answer. Thus salicylic acid is limiting and a maximum of 00305 moles 550 g of.

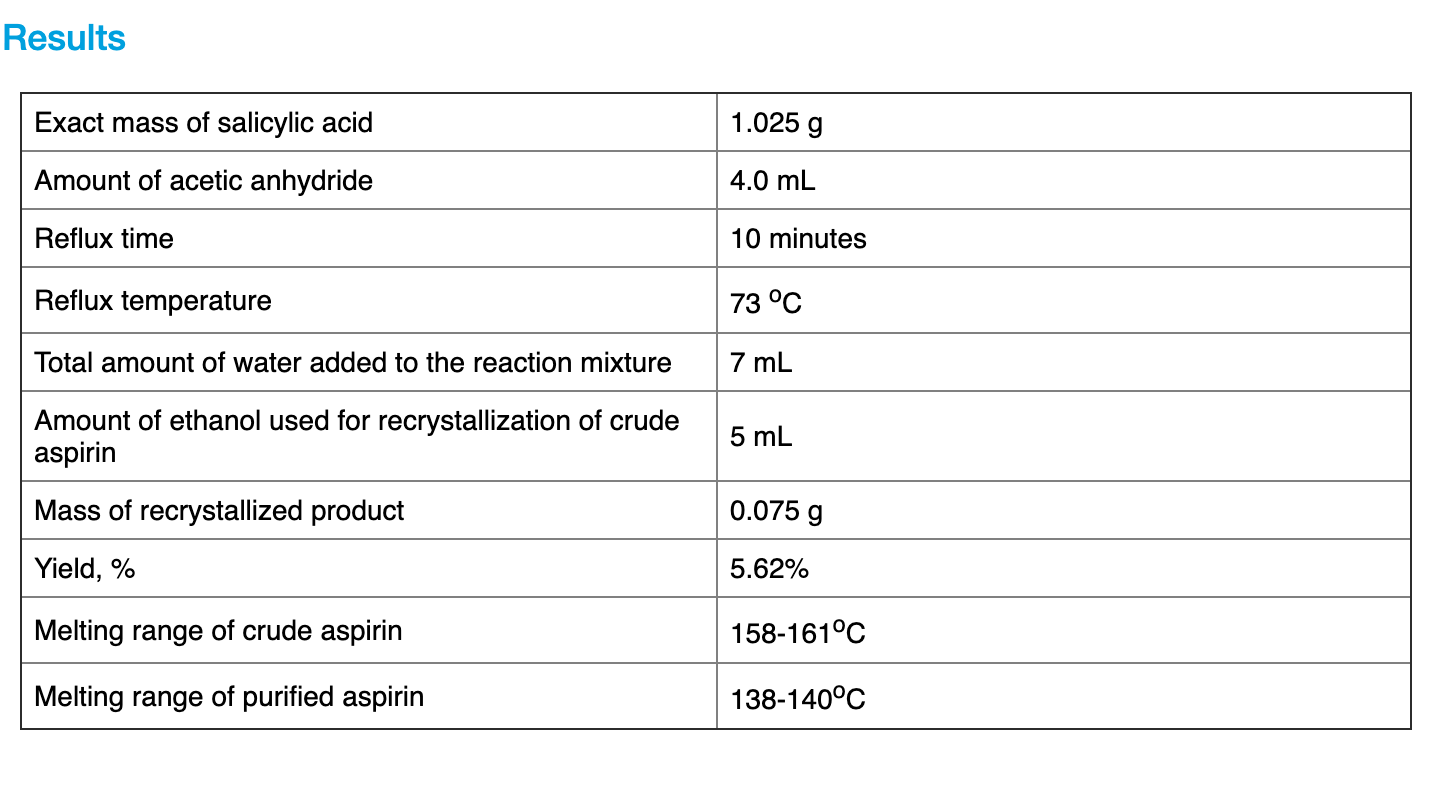
0014 moles acetylsalicylic acid 180 gmole 252 g Percent Yield experimental masstheoretical mass x 100. Where H is the distance between the tip of the spinneret and the collector h is the length of the spinneret and R is the outer radius of the spinneret. Anhydrides 2 peaks. It also provides phenol for producing caprolactam diphenylpropane medicines synthetic resins plasticizers and herbicides. The estimated answer is 400 actual answer 416.
A 747 jet is filled with 173000 L of jet fuel. All of the above 154. Calculate the molarity of a 500 ppm CaNO32 solution. -058 25 C calculated Lit Bioaccumulation is not expected. Conjugation will affect the position of the CO stretch somewhat moving it to lower wavenumber.
 Source: bartleby.com
Source: bartleby.com
Characterization of aspirin by re-crystallization and solubility. If 185 g of asp. Q163 The dipole moment ofHBr is 795 debye and the intermolecular separation is 194xlO10m Findthe ionic character in HBr molecule. Acetone for producing acetic anhydride acetonecyanhydrine and. Calculations - show your calculations below Calculation of Masses of Reactants use the volume and the literature density to calculate the mass you.
 Source: itprospt.com
Source: itprospt.com
A hydrate which has lost water is referred to as an anhydride. Compute actual molar ratio of reactants 3. C4H6O3C7H6O3C9H8O4C2H4O2In a laboratory synthesis a student begins with 500 mL of acetic anhydride density 108 g mL and 208 g of salicylic acid. Concentrations of ionic solutes are. Pure acetic acid known as glacial acetic acid is a liquid with a density of 1049 gmL at 25OC.
 Source: chegg.com
Source: chegg.com
A hydrate which has lost water is referred to as an anhydride. A hydrate which has lost water is referred to as an anhydride. Theweightof air exhausted will however be di- rectly proportional to the density and so will the static pressure developed and the horsepower consumed. For example if we find that there is lead contamination in water of 4 ppm this would mean that there are. If you see a strong peak at 1500 for example it is not CO.
 Source: chegg.com
Source: chegg.com
Acetic Anhydride Den 108 gml. The factor 13 is derived from 2 cos 493 when considering that the cone has a semivertical angle close to a possible. Therefore the theoretical yield of acetylsalicylic acid is 0014 moles. The density of acetic anhydride is 108 gmL. If 185 g of asp.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title given that the density of acetic anhydride is 108 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.