Density of triethylamine
Home » chemistry » Density of triethylamineDensity of triethylamine
Density Of Triethylamine. There are density tables of over 300 compounds built into the calculator - scroll down the page to check the list. The mechanism of the 2D rather than bulk MOF was revealed by studying the role of each component. In each group of salts the hemolytic activity increased with increased alkyl chain length. The Role of Unique Surface Chemistry Developed in Solutions Containing Fluorinated Organic Co-solvents.

One good comparison would be say triethylamine with quinuclidine. There are density tables of over 300 compounds built into the calculator - scroll down the page to check the list. The mechanism of the 2D rather than bulk MOF was revealed by studying the role of each component. The separation was performed isocratically using a mobile phase of 80 acetonitrile 15 dioxane and 5 methanolisopropyl alcohol containing 150 mM ammonium acetate and 01 triethylamine. It is not reactive at room temperatures except by strong oxidizing agents and some solvents cause swelling. Polyethylenimine600-β-cyclodextrin PEI 600-β-CyD It may be used as.
BASF Triisopropanolamine 85 Solution.
For example rates of 1-octene epoxidation increase 100-fold when the density of SiOH x increases from 0 to 5 per unit cell within Ti-BEA 5 because the disruption of H 2 O clusters near the Ti. Triethylamine is the chemical compound with the formula NCH 2 CH 3 3 commonly abbreviated Et 3 N. Specific GravityDensity11-12 30-50 Molecular FormulaSolution Molecular WeightNot available. Usually gases have a lower density than liquids because they have less cohesive particles and these in turn less than solids. Contribution of dark current density to the photodetecting properties of thieno34-bpyrazine-based low bandgap polymers Hyeokjun Kim Jinhyeon Kang Hyungju Ahn In Hwan Jung Article 109910. The one with the greatest potential for hydrogen bonding will have the highest melting point.

A programmable UVvisible detector measured carotenoids at 450 nm and a programmable fluorescence detector measured retinol at 326 nm excitation and 460 nm emission. A programmable UVvisible detector measured carotenoids at 450 nm and a programmable fluorescence detector measured retinol at 326 nm excitation and 460 nm emission. The separation was performed isocratically using a mobile phase of 80 acetonitrile 15 dioxane and 5 methanolisopropyl alcohol containing 150 mM ammonium acetate and 01 triethylamine. Last updated on. Therefore one could look at nitrogens in systems where inversion is impossible and compare their basicities to nitrogens where inversion happens.

Section 10 - Stability and Reactivity Chemical Stability. It can withstand temperatures of 80C continuously and 95 C for a short time. The nitrogen atom in DIPEA is more shielded than the nitrogen atom in triethylamine. More specifically 222-trifluoroethanol 2107 g 2106 mmol and triethylamine 1865 g 1843 mmol were added to anhydrous tetrahydrofuran THF 250 ml in. It is flexible and tough but breakable.
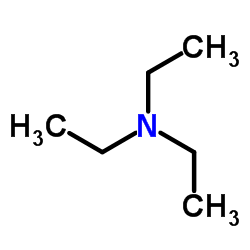 Source: chemsrc.com
Source: chemsrc.com
BASF Ultradur 4520 FC Aqua PBT. The pK a s of the respective conjugate acids in dimethyl sulfoxide are 90 and 85. LDPEs intermolecular forces are weaker its tensile strength is lower and its resilience is higher than. Another allotrope of carbon graphite has strong chemical bonds only along planes within the bulk material. Section 10 - Stability and Reactivity Chemical Stability.
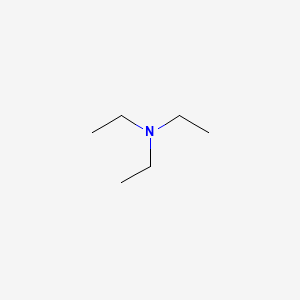
Due to their structural similarity DIPEA and triethylamine can be used interchangeably in most applications. The pkaH of triethylamine is 1075 versus 110 for quinuclidine indicating that this could indeed have a minor effect. These planes are stacked on top of each other and held together by weak. Sulfuric Acid - Density - Density of sulfuric acid at various temperatures and concentrations Water - Density Specific Weight and Thermal Expansion Coefficients - Definitions online calculator and figures and tables with water properties like density specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360C 32 to 680F. LDPEs intermolecular forces are weaker its tensile strength is lower and its resilience is higher than.
 Source: molinstincts.com
Source: molinstincts.com
It can withstand temperatures of 80C continuously and 95 C for a short time. In each group of salts the hemolytic activity increased with increased alkyl chain length. BASF E-por 002 gcc Density. Tertiary salts such as triethylamine hydrochloride produced incomplete hemolysis of dog erythrocytes in vitro whereas primary salts produced complete hemolysis. Tocol and tocopherols were.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The viscosity coefficient of triethylamine vapor over a range of density and temperature has been measured. Contribution of dark current density to the photodetecting properties of thieno34-bpyrazine-based low bandgap polymers Hyeokjun Kim Jinhyeon Kang Hyungju Ahn In Hwan Jung Article 109910. The one with the greatest potential for hydrogen bonding will have the highest melting point. Triethylamine has been used during the synthesis of. Amines are basic and can be converted to ammonium salts using.
 Source: alfachemch.com
Source: alfachemch.com
Section 10 - Stability and Reactivity Chemical Stability. ASAP High Energy Density Rechargeable Batteries Based on Li Metal Anodes. 110 Evaporation Rate10 Butyl acetate1 Viscosity. Specific GravityDensity11-12 30-50 Molecular FormulaSolution Molecular WeightNot available. The viscosity coefficient of triethylamine vapor over a range of density and temperature has been measured.

The mechanism of the 2D rather than bulk MOF was revealed by studying the role of each component. The hydrogen bonding between 2-propanol molecules is a stronger interaction and is more difficult to overcome than the dipole-dipole interactions between 2-propanone molecules. The separation was performed isocratically using a mobile phase of 80 acetonitrile 15 dioxane and 5 methanolisopropyl alcohol containing 150 mM ammonium acetate and 01 triethylamine. Triethylamine is the chemical compound with the formula NCH 2 CH 3 3 commonly abbreviated Et 3 N. The mechanism of the 2D rather than bulk MOF was revealed by studying the role of each component.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
125 cP Boiling Point. 22 nm thick via simple stirring of the reaction mixture of FeCo salts and 14-benzene dicarboxylic acid 14-BDC in the presence of triethylamine and water at room temperature. The hydrogen bonding between 2-propanol molecules is a stronger interaction and is more difficult to overcome than the dipole-dipole interactions between 2-propanone molecules. One good comparison would be say triethylamine with quinuclidine. Last updated on.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It is also abbreviated TEA yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium for which TEA is also a common abbreviation. Sulfuric Acid - Density - Density of sulfuric acid at various temperatures and concentrations Water - Density Specific Weight and Thermal Expansion Coefficients - Definitions online calculator and figures and tables with water properties like density specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360C 32 to 680F. Older subjects excreted more than younger ones. Triethylamine has been used during the synthesis of. Specific GravityDensity11-12 30-50 Molecular FormulaSolution Molecular WeightNot available.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title density of triethylamine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.