Density of sodium hydroxide
Home » chemistry » Density of sodium hydroxideDensity of sodium hydroxide
Density Of Sodium Hydroxide. 213 gcm3 20 C Melting Point. Sodium collected at the cathode. Safety Information according to GHS. 58 Monday March 26 2012 Rules and Regulations Date of issue.
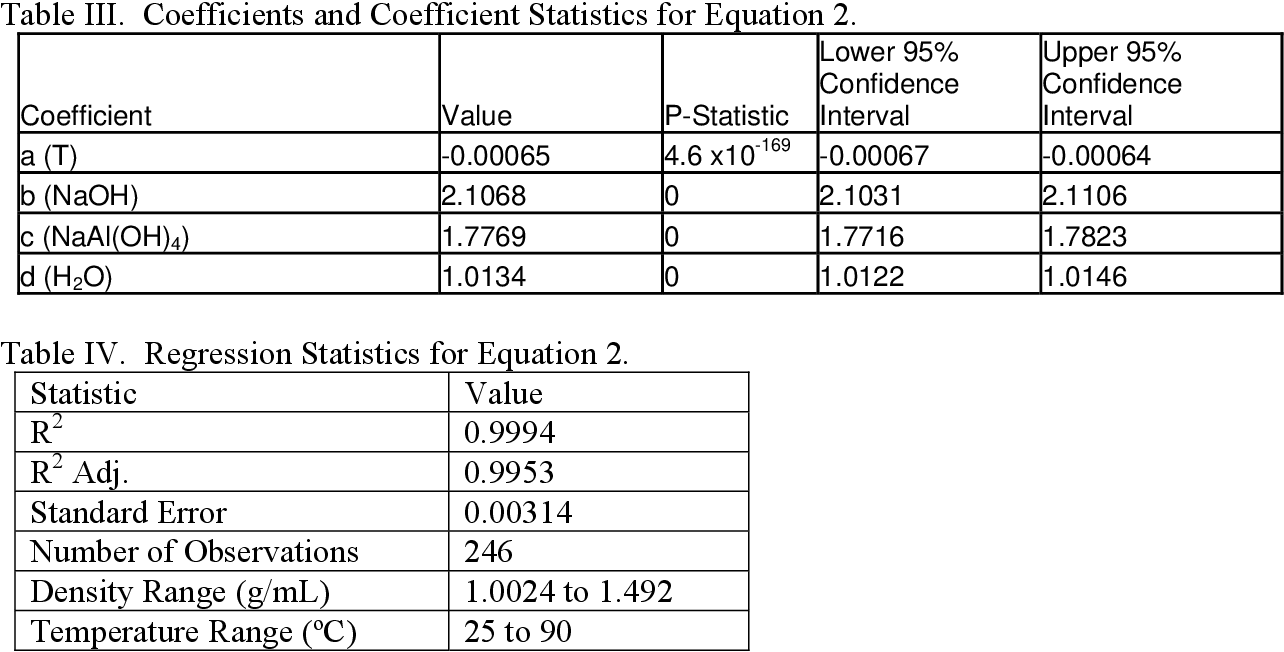 Pdf The Density Of Aqueous Sodium Hydroxide Sodium Aluminate Solutions Data Review And Model Development Semantic Scholar From semanticscholar.org
Pdf The Density Of Aqueous Sodium Hydroxide Sodium Aluminate Solutions Data Review And Model Development Semantic Scholar From semanticscholar.org
The β form of. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound. Answer 1 of 3. Identification Product form. Sodium hydroxide is a powerful and extremely corrosive alkali it decomposes living tissues. Reacts rapidly and exothermically with acids both organic and inorganic.
Sodium Hydroxide 30N 30M.
20423 was dissolved in about 25 mL of distilled water and titrated to the phenolphthalein end point with 2693 mL of a sodium hydroxide solution. It also reacts vigorously with water violently if more than a small amount of sodium meets water see video on left to produce sodium hydroxide and hydrogen gas. Sodium Hydroxide 30N 30M Safety Data Sheet according to Federal Register Vol. Sodium Hydroxide Caustic Soda. Soda Lye 1310-73-2 NaOH 960 Trade Secret Statement. OH EtOH EtO H 2 O.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Sodium Hydroxide 30N 30M Safety Data Sheet according to Federal Register Vol. It melts congruously at 755 C into a liquid with 357 NaOH and density 1392 gcm 3 and therefore floats on it like ice on water. Wear butyl rubber gloves apron andor clothing. EXPOSURE CONTROLS AND PERSONAL PROTECTION 9. Follow the OSHA.
 Source: researchgate.net
Source: researchgate.net
Shortly after Thenard and Gay-Lussac isolated sodium by reducing sodium hydroxide with iron metal at high temperatures. Relevant identified uses of the substance or mixture and uses advised against Identified uses Chemical Chemical Intermediate. Mixtures Product name. It melts congruously at 755 C into a liquid with 357 NaOH and density 1392 gcm 3 and therefore floats on it like ice on water. Sodium Hydroxide 10 wv Safety Data Sheet according to Federal Register Vol.
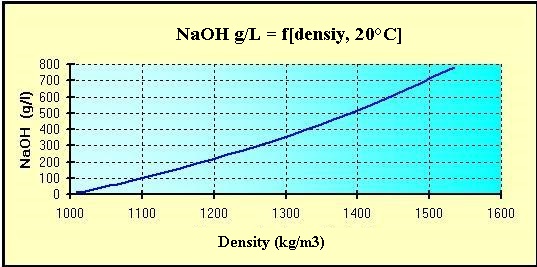 Source: hydro-land.com
Source: hydro-land.com
24 hPa 20 C Solubility. The β form of. Sodium was first isolated in 1807 by Sir Humphry Davy who made it by the electrolysis of very dry molten sodium hydroxide NaOH. The level of corrosion depends on the period of contact with the tissue and on the concentration of sodium hydroxide. The breakage of bonds in proteins may lead to severe necrosis to the application site.
 Source: yokogawa.com
Source: yokogawa.com
Reacts rapidly and exothermically with acids both organic and inorganic. Relevant identified uses of the substance or mixture and uses advised against Identified uses Chemical Chemical Intermediate. Sodium was first isolated in 1807 by Sir Humphry Davy who made it by the electrolysis of very dry molten sodium hydroxide NaOH. Sodium hydrogen sulfite sodium hydrogen tartrate sodium hydroxide sodium iodide sodium lactate sodium malate sodium maleate sodium malonate sodium metasilicate sodium metavanadate sodium molybdate sodium nitrate sodium nitrite sodium oxalate sodium perchlorate. Wear butyl rubber gloves apron andor clothing.
 Source: semanticscholar.org
Source: semanticscholar.org
10 10182013 EN English Page 1 SECTION 1. Aqueous solutions of reducing sugars other than sucrose when heated above 84C evolve toxic levels of carbon monoxide in the presence of alkalis or alkaline salts such as sodium phosphate also potassium hydroxide sodium hydroxide calcium hydroxide etc Bretherick 5th ed. The hydroxide ion by itself is not a strong enough base but it can be converted in one by adding sodium hydroxide to ethanol. Sodium hydrogen sulfite sodium hydrogen tartrate sodium hydroxide sodium iodide sodium lactate sodium malate sodium maleate sodium malonate sodium metasilicate sodium metavanadate sodium molybdate sodium nitrate sodium nitrite sodium oxalate sodium perchlorate. The pK a for self-dissociation of ethanol is about 16 so the alkoxide ion is a strong enough base.
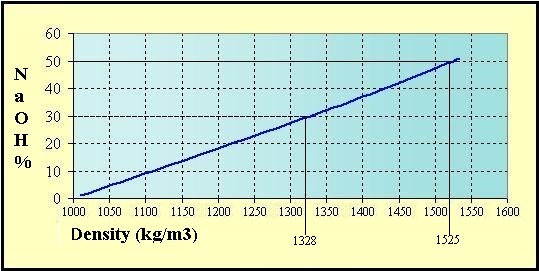 Source: hydro-land.com
Source: hydro-land.com
Most commonly solutions are 12 NaOCl with a specific gravity of 12 demonstrating that sodium hypochlorite solutions are not chemically 100 NaOCl but rather NaOCl diluted in water with salt and some sodium hydroxide left from the manufacturing process. Visit BYJUS for more information. Properties of Sodium Hydroxide. 213 Water 1 Evaporation Rate. There is a similar problem on pg 18 of your text.
 Source: researchgate.net
Source: researchgate.net
Sodium Hydroxide 30N 30M Safety Data Sheet according to Federal Register Vol. Eye contact of NaOH can cause permanent blindness. 3 A 05112-g sample of 9999 pure KHP MW. Sodium hydroxide is a powerful and extremely corrosive alkali it decomposes living tissues. Davy isolated potassium by a similar procedure also in 1807.
 Source: researchgate.net
Source: researchgate.net
3 A 05112-g sample of 9999 pure KHP MW. Sodium benzoate is produced by the neutralization of benzoic acid with caustic soda andor soda ash. 213 Water 1 Evaporation Rate. PURUMSODIUM HYDROXIDE EP PELLETSCAUSTIC SODA FLKCAUSTIC SODA MICROPEARLCAUSTIC SODA PEARL OGCAUSTIC SODA MICROPEARL SLY REACH registration number 01-2119457892-27 CAS number 1310-73-2 EU index number 011-002-00-6 EC number 215-185-5 12. Sodium benzoate structure Source PubChem What is Sodium benzoate.
 Source: researchgate.net
Source: researchgate.net
EXPOSURE CONTROLS AND PERSONAL PROTECTION 9. It melts congruously at 755 C into a liquid with 357 NaOH and density 1392 gcm 3 and therefore floats on it like ice on water. Worldwide production in 1998 was around 45 million tonnes. The density of a 50 wt NaOH solution is 153 gmL. Sodium Hydroxide Caustic Soda.
 Source: hydro-land.com
Source: hydro-land.com
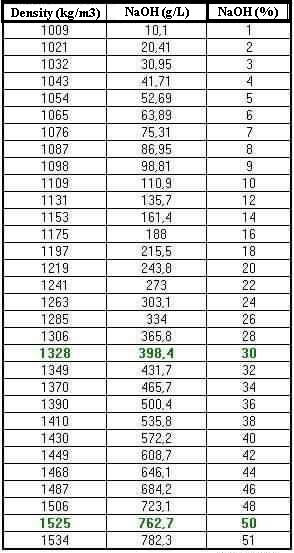
Sodium reacts with water more vigorously than lithium and less vigorously than potassium. Sodium hydroxide is a powerful and extremely corrosive alkali it decomposes living tissues. 213 gcm3 20 C Melting Point. 58 Monday March 26 2012 Rules and Regulations Date of issue. The density rating viscosity and freezing points of various sodium hydroxide concentrations are important characteristics to consider in successful bulk NaOH storage and applications.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title density of sodium hydroxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.