Density of sodium borohydride
Home » chemistry » Density of sodium borohydrideDensity of sodium borohydride
Density Of Sodium Borohydride. To the hot solution added a mixture of 234g 2 moles of. However the result is that the mercury is replaced by a hydrogen atom. The final part of the reaction sequence is displacement of mercury from the hydroxyalkylmercury complex effected through addition of sodium borohydride. Being one of the two elements in our table salt is its.
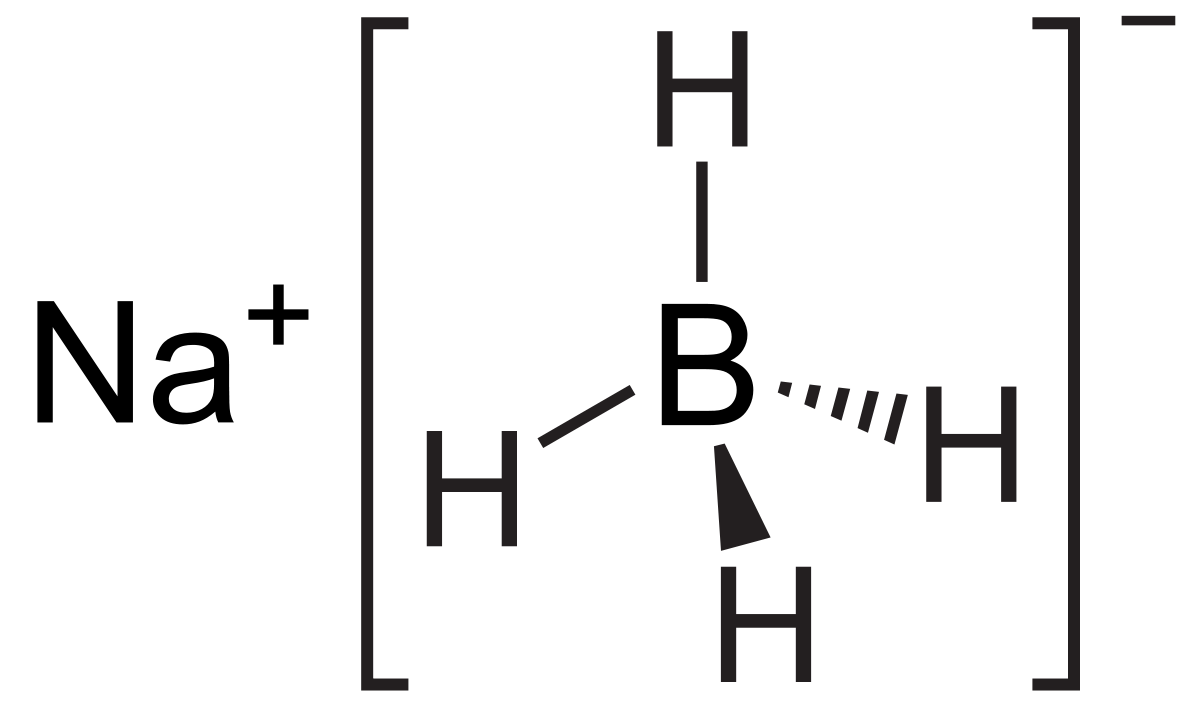
 Sodium Borohydride Formula From softschools.com
Sodium Borohydride Formula From softschools.com
ChemSpider is a free chemical structure database. Being one of the two elements in our table salt is its. Sodium borohydride is the borohydride that is produced on the largest scale industrially estimated at 5000 tonsy in 2002. Finally the as-prepared MnCoPNF electrode was washed with deionized water for several times dried and weighted. In a blatant plug for the Reagent Guide each Friday I profile a different reagent that is commonly encountered in Org 1 Org 2. The two lone pairs take equatorial positions because they demand more space than the bonds.
Reaction of sodium or potassium hydroxide on aluminum electrolysis of water or displacement from acids by certain metals.
15168 Phenyl-2-Propanone from Benzyl Cyanide. Oxidation Because it is already in a high oxidation state. Dithionite is used to bleach wood pulp. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant. Article 15 Dec 1969. 7 This approach demonstrated the potential for meeting vehicle mileage weight and volume goals.

Lithium borohydride is renowned as one of the highest energy density chemical energy carriersAlthough presently of no practical importance the solid will liberate 65 MJkg heat upon treatment with atmospheric oxygen. These specific activities were 2-3 times greater than those obtained by reduction of intact rat tail tendon collagen under similar. Reduction of a commercially available pepsin-solubilized bovine dermal collagen Vitrogen 100 PureCols old product name with sodium 3Hborohydride provided radiolabeled collagen preparations with specific activities ranging from 71-120 muCimg collagen. Acid Method for the Digestion of Gold Ore Samples. Study on The Characteristics of Nanosized Nickel Particles Using Sodium Borohydride to Promote Conversion.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A solution of sodium ethoxide is prepared from 60 g. Sodium Borohydride Nabh4 Grignard Reagent. Preparation Properties And Uses Of Caustic Soda. Finally the as-prepared MnCoPNF electrode was washed with deionized water for several times dried and weighted. Article 1 Feb 1992.
 Source: en.wikipedia.org
Source: en.wikipedia.org
UV-visible spectroscopy was used to determine the dye concentration in the. UV-visible spectroscopy was used to determine the dye concentration in the. Lithium borohydride is renowned as one of the highest energy density chemical energy carriersAlthough presently of no practical importance the solid will liberate 65 MJkg heat upon treatment with atmospheric oxygen. NaBH 4 8 NaOH 8 SO 2 4 Na 2 S 2 O 4 NaBO 2 6 H 2 O. Nathalie Godbout Dennis R.
 Source: en.wikipedia.org
Source: en.wikipedia.org
However the result is that the mercury is replaced by a hydrogen atom. Tritium is readily produced in nuclear reactors and is used in the production of the hydrogen bomb. SEM images and XRF spectra of HA and AuHA. Liquid hydrogen is important in cryogenics and in the study of superconductivity as its melting point is only 20 degrees above absolute zero. These are arranged in a trigonal bipyramidal shape with a 175 Faxial-Cl-Faxial bond angle.
 Source: softschools.com
Source: softschools.com
The reaction of the tellurium. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant. NaBH 4 8 NaOH 8 SO 2 4 Na 2 S 2 O 4 NaBO 2 6 H 2 O. Sodium borohydride NaBH 4 does not reduce carboxylic acids. Optimization of Gaussian-type basis sets for local spin density functional calculations.
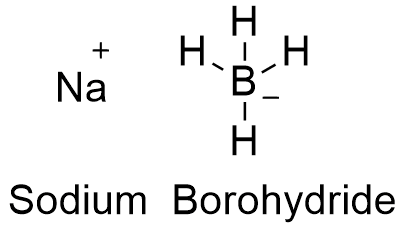 Source: softschools.com
Source: softschools.com
Being one of the two elements in our table salt is its. Sodium Borohydride Market Size will register a 67 CAGR in terms of revenue is expected to reach US 5746 million by 2026 during forecast period 2021 2026. Version 12 just got released with a host of corrections and a new page index. Tritium is readily produced in nuclear reactors and is used in the production of the hydrogen bomb. Chemoselective reductions with sodium borohydride.
 Source: sciencedirect.com
Source: sciencedirect.com
The result is a T-shaped molecule. The final part of the reaction sequence is displacement of mercury from the hydroxyalkylmercury complex effected through addition of sodium borohydride. Optimization of Gaussian-type basis sets for local spin density functional calculations. Oxidation Because it is already in a high oxidation state. These are arranged in a trigonal bipyramidal shape with a 175 Faxial-Cl-Faxial bond angle.
 Source: sciencedirect.com
Source: sciencedirect.com
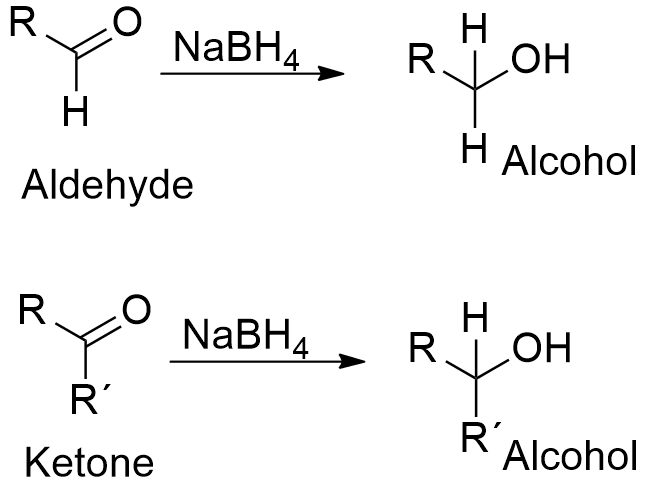
To the hot solution added a mixture of 234g 2 moles of. Article 15 Dec 1969. Sodium borohydride is also used to reduce aldehydes and ketones in the production. Optimization of Gaussian-type basis sets for local spin density functional calculations. 3 5H 2 O sodium borohydride NaBH 4 isopropanol 997 propylene oxide.
3 5H 2 O sodium borohydride NaBH 4 isopropanol 997 propylene oxide. It starts with NaBH 4 reduction of Te to sodium telluride NaHTe liberating H 2 and NaHTe in an aqueous medium with subsequent dissociation of NaHTe which releases Te anions Te 2-. The AuNPs were tested for catalytic reduction of Congo red CR a carcinogenic azo dye in aqueous sodium borohydride solution. 3 5H 2 O sodium borohydride NaBH 4 isopropanol 997 propylene oxide. Sodium Borohydride Market Size will register a 67 CAGR in terms of revenue is expected to reach US 5746 million by 2026 during forecast period 2021 2026.
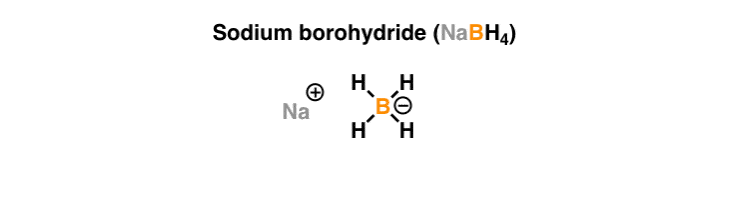 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. Acid Method for the Digestion of Gold Ore Samples. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited. Zinc Chloride Zncl2 Fe2O3. ChemSpider is a free chemical structure database.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title density of sodium borohydride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.