Density of sodium bicarbonate
Home » chemistry » Density of sodium bicarbonateDensity of sodium bicarbonate
Density Of Sodium Bicarbonate. Baking soda is a powdered chemical compound called sodium bicarbonate and vinegar includes acetic acid. Sodium sulfate and 420 mglitre for sodium bicarbonate 6. Sodium bicarbonate IUPAC name. However other modes of action have been indicated for the group of compounds commonly alluded to as buffers sodium bicarbonate potassium bicarbonate limestone magnesium oxide and bentonite Chalupa and Schneider 1985.
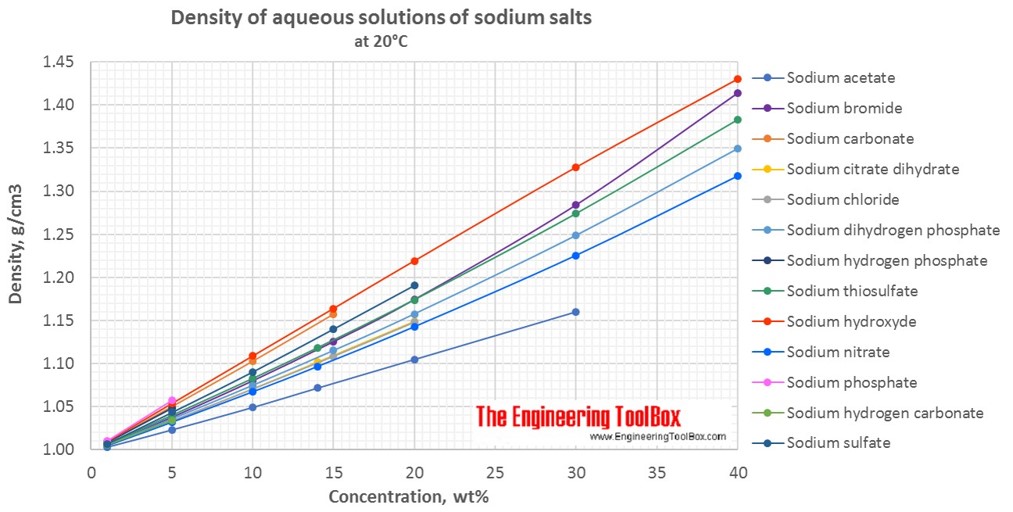 Density Of Aqueous Solutions Of Inorganic Sodium Salts From engineeringtoolbox.com
Density Of Aqueous Solutions Of Inorganic Sodium Salts From engineeringtoolbox.com
No data available Specific gravity density. These two processes yield ammonium bicarbonate and sodium chloride the double decomposition of which gives the desired sodium bicarbonate as well as ammonium chloride. 2159 gcm³ Molecular mass. 58 Monday March 26 2012 Rules and Regulations 09132019 EN English US 4. 18 26 mEqL 27 to 45 mgdL Magnesium Mg. These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below.
These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below.
The ammonia involved in the process is almost completely recovered by treating the ammonium chloride with lime to yield ammonia and. No data available. Sodium hydroxide is also known as lye or soda or caustic soda. It further absorbs carbon dioxide from the air to form sodium bicarbonate NaHCO 3. No data available Auto-ignition temperature. Relative atomic mass The mass of an atom relative to that of carbon-12.
 Source: researchgate.net
Source: researchgate.net
Density at 20 C gcm3 071 217 253. Sodium bicarbonate is a compound used for the symptomatic treatment of heartburn acid indigestion and upset stomach as well as the treatment of metabolic acidosis associated with conditions such as severe renal disease and circulatory insufficiency due to shock. Sodium is more reactive in air when in a liquid state than in a solid state. Material Lbscuft Kgscum Angle. Density is the ratio of mass to volume for a material.
 Source: sciencenotes.org
Source: sciencenotes.org
In a dry air atmosphere sodium burns by. The sodium bicarbonate is then heated to decompose it to the desired sodium carbonate. - be aware that for many of the products listed below there is a difference between bulk density and actual solid or material density. Because the bulk density of a substance varies greatly depending on how the material has been handled the information contained in this reference tool should be used for estimation purposes only. These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Major uses Metallic sodium is used in the manufacture of tetraethyl lead and sodium hydride in titanium production as a catalyst for synthetic rubber as a laboratory reagent as a coolant in nuclear reactors in electric power cables in nonglare lighting for roads and as a. These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below. The sodium bicarbonate is then heated to decompose it to the desired sodium carbonate. 18 26 mEqL 27 to 45 mgdL Magnesium Mg. Rapid correction of acidosis with sodium bicarbonate in patients with diabetic ketoacidosis may cause hypokalemia paradoxical acidosis in cerebrospinal fluid CSF since carbon dioxide diffuses more rapidly into CSF than does bicarbonate and lactic acidosis since increased pH increases hemoglobin-oxygen affinity which when combined with erythrocyte 23-diphosphoglycerate deficiency in these.

Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. The sodium blood test is often part of a basic metabolic panel. Density is the ratio of mass to volume for a material. 8401 gmol Solubility. Sodium Oxide Na2O - Sodium oxide is an inorganic compound with the chemical formula Na2O.
 Source: materials.gelsonluz.com
Source: materials.gelsonluz.com
These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below. 8401 gmol Solubility. The basic metabolic panel includes tests for. To Learn about the Physical Properties Chemical Properties and. These two processes yield ammonium bicarbonate and sodium chloride the double decomposition of which gives the desired sodium bicarbonate as well as ammonium chloride.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium Bicarbonate Safety Data Sheet according to Federal Register Vol. It further absorbs carbon dioxide from the air to form sodium bicarbonate NaHCO 3. In 2020 the global Sodium Bicarbonate market size was US 16658 million and it is expected to reach US 17614 million by the end of 2027 with a CAGR of 08 during 2021-2027. Isotopes Atoms of the same element with different. 280 to 300 mOsmkg.
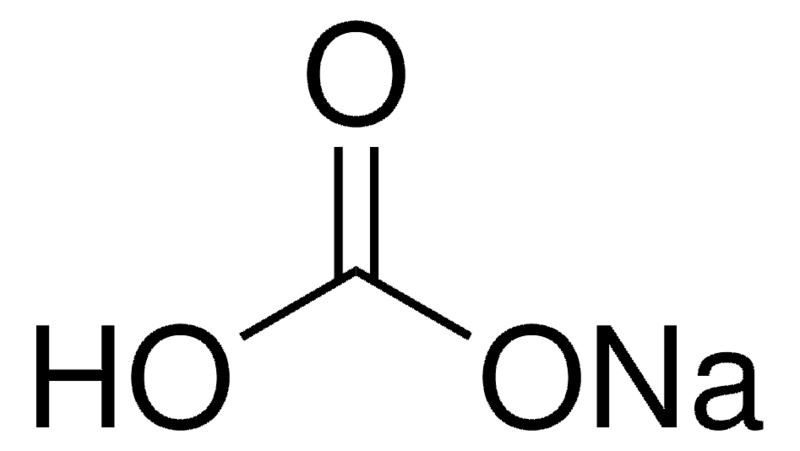 Source: sigmaaldrich.com
Source: sigmaaldrich.com
These two processes yield ammonium bicarbonate and sodium chloride the double decomposition of which gives the desired sodium bicarbonate as well as ammonium chloride. The ammonia involved in the process is almost completely recovered by treating the ammonium chloride with lime to yield ammonia and. Sodium sulfate and 420 mglitre for sodium bicarbonate 6. Ice waters solid phase is more buoyant so it forms at the surface of water bodies and freezes downward. Metallic sodium reacts with ordinary air to form a thin sodium hydroxide film NaOH.

No data available. Sodium hydroxide is also known as lye or soda or caustic soda. In a dry air atmosphere sodium burns by. Metallic sodium reacts with ordinary air to form a thin sodium hydroxide film NaOH. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH.
Sodium bicarbonate is a compound used for the symptomatic treatment of heartburn acid indigestion and upset stomach as well as the treatment of metabolic acidosis associated with conditions such as severe renal disease and circulatory insufficiency due to shock. Material Lbscuft Kgscum Angle. This Bulk Density Chart contains a searchable database of nearly 1000 products with dry powder or granular characteristics. Where more than one isotope exists the value given is the abundance weighted average. Sodium Oxide Na2O - Sodium oxide is an inorganic compound with the chemical formula Na2O.
 Source: researchgate.net
Source: researchgate.net
Incompatible with acids acidic salts dopamine hydrochloride pentazocine lactate many alkaloidal salts aspirin and bismuth salicylate. Note kgcum divided by 1602 lbscuft back to conversion home page density of liquids density of water. NaHCO3 has a white crystalline appearance. Density at 20 C gcm3 071 217 253. Incompatible with acids acidic salts dopamine hydrochloride pentazocine lactate many alkaloidal salts aspirin and bismuth salicylate.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title density of sodium bicarbonate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.