Density of naoh in water
Home » chemistry » Density of naoh in waterDensity of naoh in water
Density Of Naoh In Water. In the water molecule the. The α form of the tetrahydrate has density 133 gcm 3. Molarity amount of solute density of solution 10 molecular mass of solute or Molarity w d 10 m e. Sodium hydroxide is very corrosive.
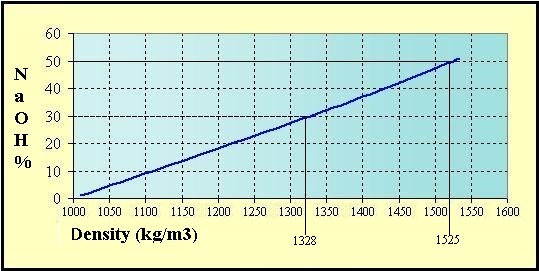 Soude From hydro-land.com
Soude From hydro-land.com
Molarity amount of solute density of solution 10 molecular mass of solute or Molarity w d 10 m e. The reaction generates heat too slowly. 1519 g cm-3 either individually or in combination with other solutions for density separation eg. Lower NaOH concentrations eg. When dissolved in water or neutralized with acid it liberates substantial heat which may be sufficient to ignite combustible materials. Sol amount of solute w 15 g Molecular mass of solute m.
You will need to dissolve 40 g of NaOH in 900 ml of distilled water and then adjust the volume to 1000 mL.
Lithium gradually reacts with water and disappears forming a colorless solution of lithium hydroxide LiOH. Therefore lithium floats on the surface of water gently fizzing and giving off hydrogen gas. A 20 mL filtered 022 μm membrane sample was diluted 15 with a distilled water previous HPLC analysis Table 111. DNA is soluble in water but insoluble in the presence of salt and alcohol. Density is given for the actual state at 25C and for liquid phase at melting point temperature. From the reviewed studies this combination may be a.
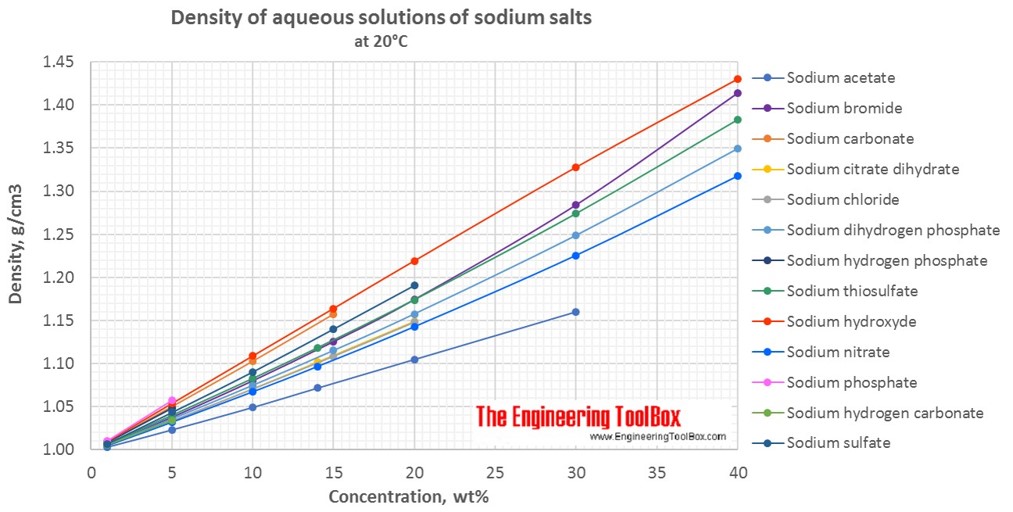 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It melts congruously at 755 C into a liquid with 357 NaOH and density 1392 gcm 3 and therefore floats on it like ice on water. By gently stirring the alcohol layer with a sterile pipette a precipitate becomes visible and can be spooled out. HClaq NaOHaq NaClaq H 2 Ol heat. Water is a solid at temperatures below 0 C a liquid between 0 C and 100 C and a gas water vapor at temperatures in excess of 100 C. Acetic acid acetic aldehyde acetone.
 Source: semanticscholar.org
Source: semanticscholar.org
The Mole Molarity and Density. First they calculate the correct volumes of 1M glucose solution and water to mix together. DNA is soluble in water but insoluble in the presence of salt and alcohol. 12 g of sodium hydroxide pellets NaOH s were dissolved in 100 mL of water at 25C. Lithium gradually reacts with water and disappears forming a colorless solution of lithium hydroxide LiOH.
 Source: researchgate.net
Source: researchgate.net
To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH. A 20 mL filtered 022 μm membrane sample was diluted 15 with a distilled water previous HPLC analysis Table 111. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Ice is less dense than water due to hydrogen bonding. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour.
 Source: researchgate.net
Source: researchgate.net
A solution of ethanolwater will have a density value which depends on the concentration of the solution. Visit BYJUS for more information. 1 M reduce the effects of NaOH on polymers but also its efficacy on OM digestion. 2Li 2H 2 O 2LiOH H 2 Sodium and water reaction. SODIUM HYDROXIDE SOLUTION refers to an aqueous solution of sodium hydroxide.
 Source: researchgate.net
Source: researchgate.net
From the reviewed studies this combination may be a. SODIUM HYDROXIDE SOLUTION refers to an aqueous solution of sodium hydroxide. Currently sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and convert it to the salt. It is generally used as a solid or a 50 solution. If there is lots of DNA you may see a stringy white precipitate.
 Source: hydro-land.com
Source: hydro-land.com
If there is lots of DNA you may see a stringy white precipitate. Air bubbles added to a molten soap will decrease the density of the soap and thus it will float on water. Water is a solid at temperatures below 0 C a liquid between 0 C and 100 C and a gas water vapor at temperatures in excess of 100 C. Liquid water also shows an exception to this rule from 0 degrees Celsius to 4 degrees Celsius where it increases in density instead of decreasing as expected. A solution of ethanolwater will have a density value which depends on the concentration of the solution.
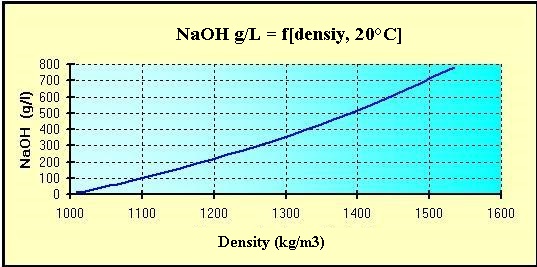 Source: hydro-land.com
Source: hydro-land.com
Other common names include caustic soda and. The temperature of the water rose to 275C. Since the solutions are mostly water the solutions are assumed to have a density of 10 gmL and a specific heat of 418 JgC. A 20 mL filtered 022 μm membrane sample was diluted 15 with a distilled water previous HPLC analysis Table 111. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour.
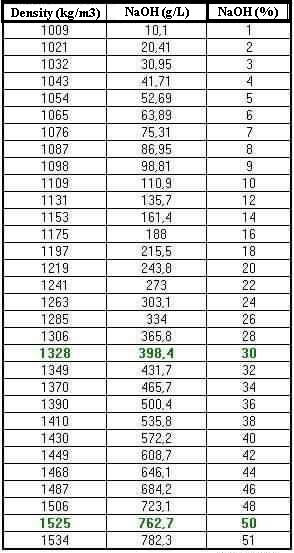 Source: hydro-land.com
Source: hydro-land.com
HClaq NaOHaq NaClaq H 2 Ol heat. Looking at the table you can also see that ice is less dense than water. It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. If there is lots of DNA you may see a stringy white precipitate. Calculate the heat released q in joules J by the reaction.
 Source: yokogawa.com
Source: yokogawa.com
Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. Density of aqueous solutions assumed to be the same as for water 1 g mL-1 at 25C example. The reaction generates heat too slowly. Molarity amount of solute density of solution 10 molecular mass of solute or Molarity w d 10 m e. This can easily be observed in a water-filled bath or.
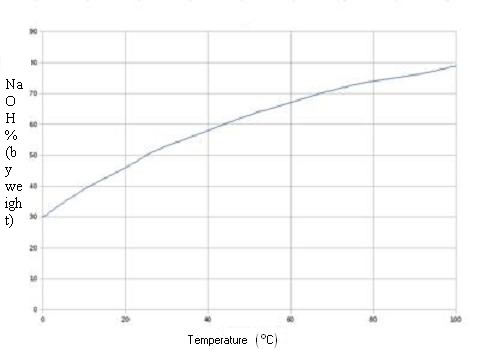 Source: hydro-land.com
Source: hydro-land.com
Answer 1 of 7. Sodium hydroxide is very corrosive. Lower NaOH concentrations eg. DNA is soluble in water but insoluble in the presence of salt and alcohol. Lithium gradually reacts with water and disappears forming a colorless solution of lithium hydroxide LiOH.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title density of naoh in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.