Density of hydrogen peroxide solution
Home » chemistry » Density of hydrogen peroxide solutionDensity of hydrogen peroxide solution
Density Of Hydrogen Peroxide Solution. Hydrogen peroxide - urea also called Hyperol artizone urea hydrogen peroxide and UHP is a solid composed of equal amounts of hydrogen peroxide and ureaThis compound is a white crystalline solid which dissolves in water to give free hydrogen peroxide. 1 Mass of 1 liter of solution. 58 Monday March 26 2012 Rules and Regulations 07122017 EN English US 59 Relative vapor density at 20 C. 125 ml ethanol in approx.
 The Behaviour Of Mixtures Of Hydrogen Peroxide And Water Part 1 Determination Of The Densities Of Mixtures Of Hydrogen Peroxide And Water Semantic Scholar From semanticscholar.org
The Behaviour Of Mixtures Of Hydrogen Peroxide And Water Part 1 Determination Of The Densities Of Mixtures Of Hydrogen Peroxide And Water Semantic Scholar From semanticscholar.org
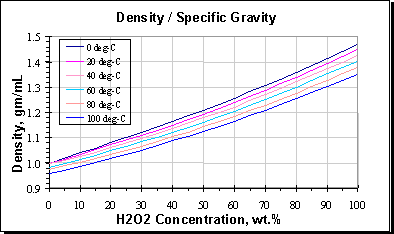
The hydrogen peroxide used for this demonstration is four times stronger than the over-the-counter variety you can buy at the store. Emergency eye wash fountains and safety showers should be available in the immediate vicinity of usehandlingProvide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapor or mists. For L-lactate detection the functionalized biosensor works in two linear regimes. Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. This is the list of all compounds with density tables present in the full database - it was actual on May 4 th 2005 as the list is growing it may be already incomplete. 10 ww H 2 O 2 10 g hydrogen peroxide in 90 gm 90 ml water.
So use the density of the solution to determine how many grams you get in that respective volume 100colorredcancelcolorblackL.
The solution is then mixed with potassium iodide and the iodide formed is titrated with 01 N sodium thiosulfate with starch indicator. Personal Protective Equipment It is the responsibility of the employer to determine the potential risk of exposure to hazardous chemicals for employees in the workplace in order to. For L-lactate detection the functionalized biosensor works in two linear regimes. We found that although some surfaces were softened no enamel erosion was found. It is a strong oxidising agent and is particularly effective for the cleaning of wounds cuts and other damaged tissue portions. Engineering measures to reduce exposure.
 Source: semanticscholar.org
Source: semanticscholar.org
We found that although some surfaces were softened no enamel erosion was found. Or 15 parts by weight of salt NaCl in 85 parts by weight of water 15 ww 125 o C was the original definition of 15 o Buame. The strongest hydrogen peroxide solutions such as 30 32 34 and 35 are normally diluted before. Please see the certificate of analysis for the recommend retest date for a particular lot of Product No. Manufacture of hydrogen peroxide H₂O₂ Hydrogen peroxide is a routine sterilising agent used in clinics and hospitals.
 Source: topologicoceans.wordpress.com
Source: topologicoceans.wordpress.com
Check our FAQ section for more details. The influence of the strong hydrogen bonds of the dye with three water molecules on the absorption spectrum was analyzed. Hydrogen Peroxide 30 ww Safety Data Sheet according to Federal Register Vol. The hydrogen peroxide found at the grocery store is a 3 solution which is safe to touch yet is powerful enough to kill bacteria viruses and fungi on surfaces. It is also used for bleaching hair whitening teeth and removing.

1 Mass of 1 liter of solution. It was found that explicit assignment of water molecules. The first thing to do here is pick a sample of this solution. The main uses of hydrogen peroxide are in the preparation of other peroxides and as an oxidising agent. It is a colourless liquid and is used in aqueous solution for safety reasons.
 Source: thevespiary.org
Source: thevespiary.org
Although dilute solutions of hydrogen peroxide have a limited shelf life the 30 solution when properly stored has a shelf life measured in years. Hydrogen peroxide H 2 O 2 is an important green oxidant 1 widely used in a variety of industries and a promising clean fuel for jet car and rockets 234567 60 wt H 2 O 2 has an energy. 10 ww H 2 O 2 10 g hydrogen peroxide in 90 gm 90 ml water. 141 gcm³ Solubility. The hydrogen peroxide found at the grocery store is a 3 solution which is safe to touch yet is powerful enough to kill bacteria viruses and fungi on surfaces.
 Source: chemistry.mdma.ch
Source: chemistry.mdma.ch
Hydrogen peroxide exerts cerumenolytic enamel-bleaching and antiseptic actions. The hydrogel is functionalized with lactate oxidase which catalyzes the oxidation of L-lactate to pyruvate forming hydrogen peroxide. Concentrated hydrogen peroxide is a very reactive oxygen species and is used as a propellant in rocketry. Maps of the distribution of electron density and electrostatic potential have been built. 1 4 mgm3 Appropriate Engineering controls.
 Source: semanticscholar.org
Source: semanticscholar.org
Personal Protective Equipment It is the responsibility of the employer to determine the potential risk of exposure to hazardous chemicals for employees in the workplace in order to. A solution of hydrogen peroxide H 2 O 2 is 300 by mass and has a density of 111 gcm 3Calculate the a molality b molarity and c mole fraction Solution. The chemical formula for hydrogen peroxide is H 2 O 2. No data available Specific gravity density. Low percentage hydrogen peroxide 3 is great at.
 Source: en.wikipedia.org
Source: en.wikipedia.org
10 ww H 2 O 2 10 g hydrogen peroxide in 90 gm 90 ml water. Hydrogen peroxide is a chemical compound with the formula H 2 O 2In its pure form it is a very pale blue liquid slightly more viscous than waterIt is used as an oxidizer bleaching agent and antiseptic usually as a dilute solution 36 by weight in water for consumer use and in higher concentrations for industrial useConcentrated hydrogen peroxide or high-test peroxide. Hydrogen peroxide is the simplest kind of peroxide available oxygen-oxygen single bond. One more dilution will produce a solution that is approximately 35. The main uses of hydrogen peroxide are in the preparation of other peroxides and as an oxidising agent.
 Source: researchgate.net
Source: researchgate.net
Hydrogen peroxide - urea also called Hyperol artizone urea hydrogen peroxide and UHP is a solid composed of equal amounts of hydrogen peroxide and ureaThis compound is a white crystalline solid which dissolves in water to give free hydrogen peroxide. One more dilution will produce a solution that is approximately 35. The molarity of a 30 hydrogen peroxide solution is 98 M based on a density of 111 gml. Or 15 parts by weight of salt NaCl in 85 parts by weight of water 15 ww 125 o C was the original definition of 15 o Buame. Manufacture of hydrogen peroxide H₂O₂ Hydrogen peroxide is a routine sterilising agent used in clinics and hospitals.
 Source: h2o2.com
Source: h2o2.com
It is possible to purchase various concentrations of hydrogen. In aqueous solution it has been experimentally and theoretically reported that adsorbed OH is stabilized by 0408 eV by hydrogen bonding interactions with nearby H 2 O molecules 414243. It is a strong oxidising agent and is particularly effective for the cleaning of wounds cuts and other damaged tissue portions. Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. Personal Protective Equipment It is the responsibility of the employer to determine the potential risk of exposure to hazardous chemicals for employees in the workplace in order to.
 Source: semanticscholar.org
Source: semanticscholar.org
To make four different solutions start with 30 hydrogen peroxide and dilute it by one-half to make a 15 solution. Figure 6 Low pressure gas-phase process. Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. Now you have the four solutions needed for the experiment. The five products included strips at 65 hydrogen peroxide and gels at 10 and 22 carbamide peroxide 35 hydrogen peroxide and a sodium hypochlorite containing gel system.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title density of hydrogen peroxide solution by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.