Density of hcl solution
Home » chemistry » Density of hcl solutionDensity of hcl solution
Density Of Hcl Solution. 1187 gmL -849 C Kaye Laby No longer updated 203 gmL -160 C Kaye Laby No longer updated Miscellaneous. Therefore we can directly substitute HCl concentration to pH equation. A solution containing 42 NaOH by mass has a density of 130 gmL. Hydrogen chloride - 2-propanol solution 125 M HCl T for GC derivatization 185912-82-7 Chloride atomic spectroscopy standard concentrate 1000 g Cl- 1000 gL.
 Hcl From hydro-land.com
Hcl From hydro-land.com
Record the molarity of the HCl used. Creating a Stock Solution Autograded Virtual Lab. To prepare 1000 mL of a 01 molL solution of AlCl3 we have to dissolve 241433 g of AlCl36H2O 96 purity in deionized or distilled water. 148 gmlMolecular weight of HCl. Samples containing dissolved organic gas eg. 320 is 35 of 900 hence the statement it is is a about a 40 solution So the volume that would have 125 grams would be 125320 L 039 L 39 mL.
Butane show a tendency to degas so they should be cooled down to avoid bubble generation.
Add 826 mL of concentrated HCl to about 50 mL of distilled water stir then add water up to 100 mL. Hydrochloric acid abbreviation HCl aq is a common acid both in the body and in the labIt is for example a major component of gastric acid pH 1-2 05 wv HCl. Percent Composition Problem Solutions. The Mole Molarity and Density. An aqueous solution is 400 by mass hydrochloric acid HCl and has a density of 120 gmL. The Spectrophotometer helps measure the absorption spectrum the absorption of light by a solution at each wavelength.

What is its molar concentration. Hydrogen chloride - 2-propanol solution 125 M HCl T for GC derivatization 185912-82-7 Chloride atomic spectroscopy standard concentrate 1000 g Cl- 1000 gL. To 200 L of 0445 M HCl you add 388 L of a second HCl solution of an unknown concentration. Samples containing dissolved organic gas eg. It is the basic Principle of spectrophotometry in biochemistry.
 Source: youtube.com
Source: youtube.com
08-0811 gmL 20 C Sigma-Aldrich SIAL-07607. Tris and to reveal antigens eg. Percent Solutions Mass percent solutions are defined based on the grams of solute per 100 grams of solution. Colorless to slightly yellow gas with a pungent irritating odor. See the Related Questions for complete instructions on how to prepare a solution by diluting a stock solution.
 Source: wiki.anton-paar.com
Source: wiki.anton-paar.com
An aqueous solution is 400 by mass hydrochloric acid HCl and has a density of 120 gmL. HYDROCHLORIC ACID HCL 920 SATURATED LIQUID DENSITY Temperature degrees F Pounds per cubic foot 40 50 60 70 80 90 100 110 120 74770 74599 74419 74250 74080 73900 73730 73559 73381 921 LIQUID HEAT CAPACITY Temperature degrees F British thermal unit per pound-F 35 40 45 50 55 60 65 70 75 80 85 90 95 100 105 110 115. 20 g of sodium chloride in 100 g of solution is a 20 by mass solution. If concentrated acids or bases eg. OU Chemical Safety Data No longer updated More details.
 Source: mt.com
Source: mt.com
What mass in kilograms of NaOH is in 600 L of this solution. A solution of water and HCl contains 25 HCl by mass. 4522cancelg 1 mole3646cancelg 1240 moles Since the sample has a volume of 100-L the molarity. 20 g of sodium chloride in 100 g of solution is a 20 by mass solution. 08-0811 gmL 20 C Sigma-Aldrich SIAL-07607.
 Source: youtube.com
Source: youtube.com
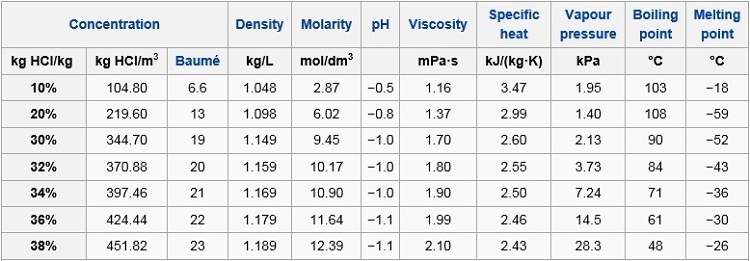
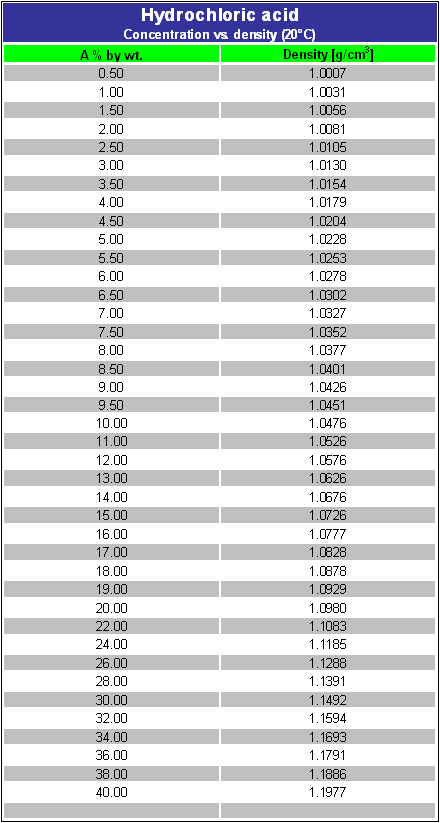
Density of the HCl solution g cm-3 HCl ww Calculate pH. To prepare 1000 mL of a 01 molL solution of AlCl3 we have to dissolve 241433 g of AlCl36H2O 96 purity in deionized or distilled water. Properties apply to 37 solution. This material contains Hydrogen chloride CAS 7647-01-0 32-38which is subject to the reporting requirements of Section 313 of SARA Title III and 40 CFR Part 373. Percent Solutions Mass percent solutions are defined based on the grams of solute per 100 grams of solution.
 Source: researchgate.net
Source: researchgate.net
CsF is 875 Cs and 125 F by mass. Volume percent solutions are defined as milliliters of solute per 100 mL of solution. Since the density is 1320 gL then for every L of solution there is 320 grams of solute. Calculate pH of HCl using pH equation. This example has neither the moles nor liters needed to find molarity so you must find the number of moles of the solute first.
 Source: researchgate.net
Source: researchgate.net
After the solid is completely dissolved dilute the solution to a final volume with deionized distilled water. If concentrated acids or bases eg. H 2 SO 4 HCl NaOH are measured minimize contact and evaporation by using an automated system with an autosampler. Hydrogen chloride - 2-propanol solution 125 M HCl T for GC derivatization 185912-82-7 Chloride atomic spectroscopy standard concentrate 1000 g Cl- 1000 gL. To convert volume to mass we need the density of the solution.
 Source: hydro-land.com
Source: hydro-land.com
1187 gmL -849 C Kaye Laby No longer updated 203 gmL -160 C Kaye Laby No longer updated Miscellaneous. International Starch Institute Science Park Aarhus Denmark Specific Density of Hydrochloric Acid Solutions. Normally we dont weigh liquid solutions we measure the volume. Butane show a tendency to degas so they should be cooled down to avoid bubble generation. 1190cancelg solution 38 g HCl100cancelg solution 4522 g HCl To determine the solutions molarity use hydrochloric acids molar mass - this will get you the number of moles of acid present in the sample.

Calculate pH of HCl using pH equation. HYDROCHLORIC ACID HCL 920 SATURATED LIQUID DENSITY Temperature degrees F Pounds per cubic foot 40 50 60 70 80 90 100 110 120 74770 74599 74419 74250 74080 73900 73730 73559 73381 921 LIQUID HEAT CAPACITY Temperature degrees F British thermal unit per pound-F 35 40 45 50 55 60 65 70 75 80 85 90 95 100 105 110 115. Normally we dont weigh liquid solutions we measure the volume. The density of the solution is 105 gmL. See the Related Questions for complete instructions on how to prepare a solution by diluting a stock solution.
 Source: youtube.com
Source: youtube.com
Density of the HCl solution g cm-3 HCl ww Calculate pH. The Mole Molarity and Density. What mass in kilograms of NaOH is in 600 L of this solution. Since the density is 1320 gL then for every L of solution there is 320 grams of solute. Clear colourless or slightly yellow liquid with pungent odour.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title density of hcl solution by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.