Density of dichloromethane
Home » chemistry » Density of dichloromethaneDensity of dichloromethane
Density Of Dichloromethane. Dipole moments compare less favorably. 000 C 3200 F. If not breathing give artificial respiration. 09167 gml at 0 C.
 Physical Properties Of Dichloromethane Download Table From researchgate.net
Physical Properties Of Dichloromethane Download Table From researchgate.net
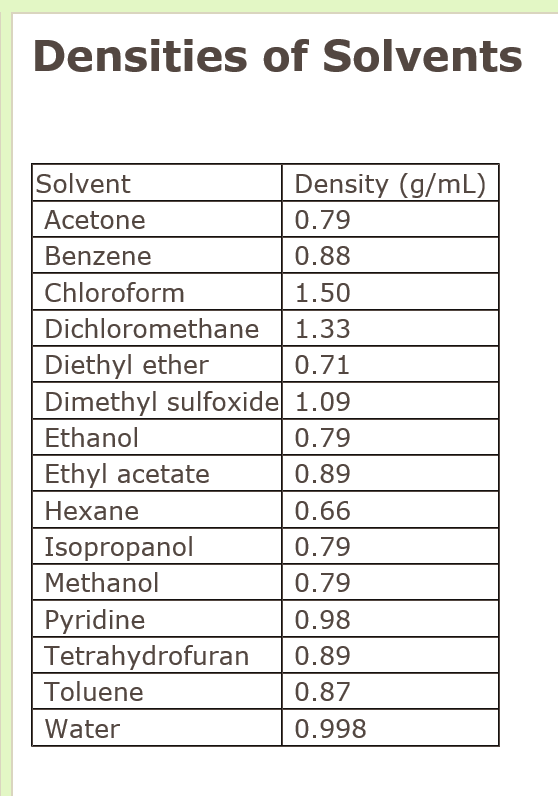
Density is defined as the mass per unit volume of a substance. For extraction you will observe a significant build up of pressure as well. Although there are exceptions usually increasing the temperature decreases density. Limits Density ppm Air 1 Common Name Other Names LEL UEL Acetone 0 134 26 128 20 1000 Benzene Benzol 12 176 13 71 28 1 Carbon Disulfide Carbon bisulfide -22 115 13 500 26 20 12- Dichloroethylene Acetylene dichloride 43 140 97 128 34 200 Ethyl Acetate 24 171 22 110 30 400 Ethyl Alcohol Ethanol 55 173 33 19 16 1000 Grain alcohol Ethyl Benzene 59 277 10 67 37 100. The solubilities of caffeine as a function of tempera-ture in water and ethanol in this work were compared. 3bc and the relevant photovoltaic parameters including V oc J sc and FF are summarized in Table 2.
Limits Density ppm Air 1 Common Name Other Names LEL UEL Acetone 0 134 26 128 20 1000 Benzene Benzol 12 176 13 71 28 1 Carbon Disulfide Carbon bisulfide -22 115 13 500 26 20 12- Dichloroethylene Acetylene dichloride 43 140 97 128 34 200 Ethyl Acetate 24 171 22 110 30 400 Ethyl Alcohol Ethanol 55 173 33 19 16 1000 Grain alcohol Ethyl Benzene 59 277 10 67 37 100.
Replacing dichloromethane is advantageous when conditions require higher boiling solvents since trifluorotoluene boils 62 C higher than. Relative density water 1. 1322 at 68 F USCG 1999 Boiling. Lide ed CRC Press Boca Raton FL 2004 except as noted. Laboratory chemical Identification of Manufacturer. Density of DCM.
 Source: chegg.com
Source: chegg.com
35061011 Use of chemical. Research Triangle Park North Carolina. If not breathing give artificial respiration. 09998396 gmL at 0 C. Although there are exceptions usually increasing the temperature decreases density.
 Source: researchgate.net
Source: researchgate.net
Melting point of DCM-976 0 C. LEL 130000 ppm DOE 2016 Regulatory Information. Water has a density of 1 kgl at 4C. 09970474 gmL at 25 C. Usually gases have a lower density than liquids because they have less cohesive particles and these in turn less than solids.

1765 K Boiling point. The dielectric constants for dichloromethane and trifluorotoluene are 904 and 918 respectively indicating similar solvating properties. The Regulatory Information fields include information from the US. Limits Density ppm Air 1 Common Name Other Names LEL UEL Acetone 0 134 26 128 20 1000 Benzene Benzol 12 176 13 71 28 1 Carbon Disulfide Carbon bisulfide -22 115 13 500 26 20 12- Dichloroethylene Acetylene dichloride 43 140 97 128 34 200 Ethyl Acetate 24 171 22 110 30 400 Ethyl Alcohol Ethanol 55 173 33 19 16 1000 Grain alcohol Ethyl Benzene 59 277 10 67 37 100. Boiling point of DCM.

Molecular weight of DCM. 1322 at 68 F USCG 1999 Boiling. 1617 NTP 1992 Relative to Air National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Product and Company Identification Product Identifier. VWR International 1310 Goshen Parkway West Chester PA 19380 FOR MORE INFORMATION CALL.

Relative Density 15189 9591507 992 at 20C. Dichloromethane is a member of the class of chloromethanes that is methane in which two of the hydrogens have been replaced by chlorineA dense non-flammible colourless liquid at room temperature bp. Sulfuric Acid - Density - Density of sulfuric acid at various temperatures and concentrations Water - Density Specific Weight and Thermal Expansion Coefficients - Definitions online calculator and figures and tables with water properties like density specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360C 32 to 680F. Physiochemical Properties of the Technical Grade Test Compound Parameter Value Water solubility 20C Deionized Water 1023 mgL pH 4 0972 mgL pH 7 0880 mgL pH 9 0971 mgL Solvent solubility 20C Acetone 3446 0172 gL Acetonitrile 0711 0072 gL Ethyl Acetate 1144 0046 gL Dichloromethane 2476 0. Although there are exceptions usually increasing the temperature decreases density.
 Source: chem.libretexts.org
Source: chem.libretexts.org
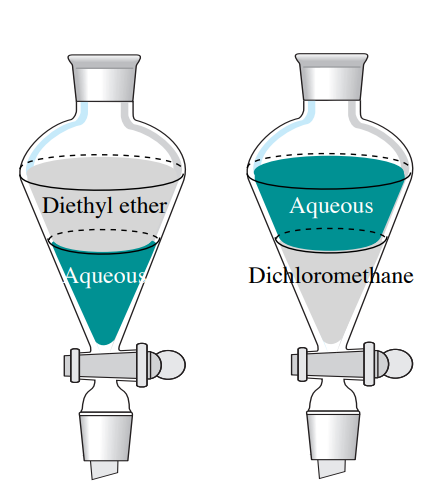
Although there is a difference between specific. As the compound is. It has been used as the principal component of paint stripper although replacements exist. Propane - Density and Specific Weight vs. One rule that should always be followed when performing a work-up process.
It has been used as the principal component of paint stripper although replacements exist. The currentdensity-voltage J-V curves and external quantum efficiency EQE plots of the optimized devices are depicted in Fig. 40 d 133 which is immiscible with water it is widely used as a solvent a paint stripper and for the removal of caffeine from coffee and tea. Note that in almost every case one of the solvents is water or an aqueous solution. Improved solubility in amines ketones.
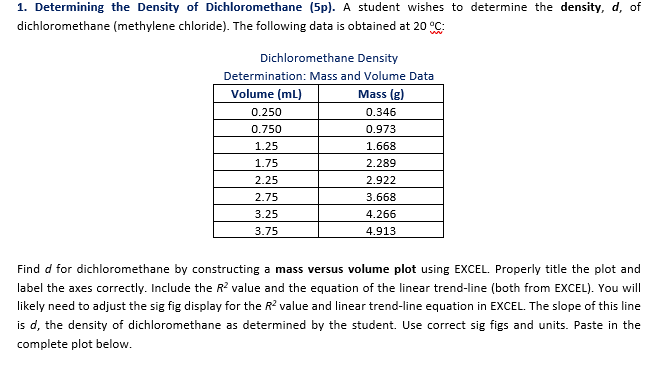 Source: chegg.com
Source: chegg.com
Dipole moments compare less favorably. The exceptionally low trap density found experimentally can be explained with the aid of density functional theory DFT calculations performed on MAPbBr 3 which predict a high formation energy for deep trap defects when MAPbBr 3 is synthesized under Br-rich conditions eg from PbBr 2 and MABr such as is the case in this study. Inhalation Remove to fresh air. Replacing dichloromethane is advantageous when conditions require higher boiling solvents since trifluorotoluene boils 62 C higher than. LEL 130000 ppm DOE 2016 Regulatory Information.
 Source: clutchprep.com
Source: clutchprep.com
Density itself is defined as how much mass a substance has in a specific volume. 3bc and the relevant photovoltaic parameters including V oc J sc and FF are summarized in Table 2. It equals centipoise divided by specific density. Research Triangle Park North Carolina. Propane - Density and Specific Weight vs.

0961893 gmL at 95 C. Lide ed CRC Press Boca Raton FL 2004 except as noted. DCM is used as a solvent in the food industry and as a paint remover. Replacing dichloromethane is advantageous when conditions require higher boiling solvents since trifluorotoluene boils 62 C higher than. 09970474 gmL at 25 C.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title density of dichloromethane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.