Density of bromoethane
Home » chemistry » Density of bromoethaneDensity of bromoethane
Density Of Bromoethane. 1003 to 1018 F. It decomposes thermally via the following chemical reactions. Includes kit list and safety instructions. Try this practical or demonstration to produce bromoethane in a substitution reaction between ethanol and phosphorus tribromide.
 Bromoethane D5 99 Atom D 3675 63 6 From sigmaaldrich.com
Bromoethane D5 99 Atom D 3675 63 6 From sigmaaldrich.com
Miscible with ethanol ether. It has a role as a carcinogenic agent a solvent a refrigerant a local anaesthetic and an alkylating agent. Solomon Derese 18 Free Radical Reactions The term free radical refers to any atom or group of atoms with an odd number of electrons. Br KCN CH. Sodium propionate from iodomethane methyl iodide and bromoethane ethyl bromide. Bromoethane is a bromoalkane that is ethane carrying a bromo substituent.
A dry mixture of KOH K_2CO_3 and KCl weighing.
Figure 42 - Effect of the partial pressure of hydrogen on the free energy of conversion of. Fluid Mechanics - The study of fluids - liquids and gases. The electron density map for ethene below shows the region of high electron density red clearly and can be compared with the electron density map for alkanes to see why alkenes are reactive while alkanes are inert and why the attacking species approaches the alkene side-on. Try this practical or demonstration to produce bromoethane in a substitution reaction between ethanol and phosphorus tribromide. Most alkene reactions involve breaking the π-bond which has a lower bond enthalpy. Other mutants resistant to DL-ethionine or 2-bromoethane sulfonate coenzyme M analogue in addition to autotrophic mutants were obtained by mutagenic treatment.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
51 C and 0. The funnel is stoppered and the mixture is shaken vigorously. Br KCN CH. Flammable substance - fuel. Includes kit list and safety instructions.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Flammable substance - fuel. The cornflour bomb In association with Nuffield Foundation. 1-Bromoethane 1-Bromopropane 1-Bromobutane Bromobenzene a Bromobenzene 1-Bromobutane 1-Bromopropane 1-Bromoethane b Bromobenzene 1-Bromoethane 1-Bromopropane 1-Bromobutane c 1-Bromopropane 1-Bromobutane 1-Bromoethane Bromobenzene d 1-Bromoethane 1-Bromopropane 1-Bromobutane Bromobenzene. Source of ignition - spark or high heat. Change the bond angle to see how shape affects polarity.

After standing two layers separate. Arrange the following compounds in increasing order of their boiling. This leaves a partial positive charge on the carbon atom making it a reactive center. 1 Outline the mechanism for this reaction. Last updated on.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Includes kit list and safety instructions. It has a role as a carcinogenic agent a solvent a refrigerant a local anaesthetic and an alkylating agent. A Into a separatory funnel is poured 50 mL of CH3CH2Br bromoethane a water-insoluble compound with a density of 1460 gmL and 50 mL of water. Give your answer to the appropriate number of significant figures. A In bromoethane and chloroethane mixture intermolecular interactions of A-A and B-B types are nearly same as A-B type interactions.
 Source: fishersci.se
Source: fishersci.se
This time we are doing a secondary alcohol. 1 Outline the mechanism for this reaction. The Flammable Range also called Explosive Range is the concentration range of a gas or vapor that will burn or explode if an ignition source is introduced. Source of ignition - spark or high heat. 3 Calculate the percentage atom economy for the formation of D in this reaction.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
B In ethanol and acetone mixture A-A or B-B type intermolecular interactions are stronger than A-B type interactions. Last updated on. A dry mixture of KOH K_2CO_3 and KCl weighing. Figure 42 - Effect of the partial pressure of hydrogen on the free energy of conversion of. Sodium AcetateCH3COONa- Sodium acetate is the salt of acetic acid and sodium hydroxide.
 Source: wikidata.org
Source: wikidata.org
Bromoethane reacts with potassium cyanide to form compound. 1-Bromoethane 1-Bromopropane 1-Bromobutane Bromobenzene a Bromobenzene 1-Bromobutane 1-Bromopropane 1-Bromoethane b Bromobenzene 1-Bromoethane 1 -Bromopropane 1-Bromobutane c 1-Bromopropane 1-Bromobutane 1-Bromoethane Bromobenzene d 1-Bromoethane 1-Bromopropane 1-Bromobutane Bromobenzene Solution. A Into a separatory funnel is poured 50 mL of CH3CH2Br bromoethane a water-insoluble compound with a density of 1460 gmL and 50 mL of water. 1 mark 0 2. So bromoethane as our final result.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Flammable substance - fuel. Sodium propionate from iodomethane methyl iodide and bromoethane ethyl bromide. Brines High Density ZnBr Brines Low Density CaNaCl Bromic Acid. Oxidizer - oxygen or air. The melting points of butanoic acid bromoethane and dimethyl ether are below 25 25 however the boiling points of all three molecules are above 25 25.
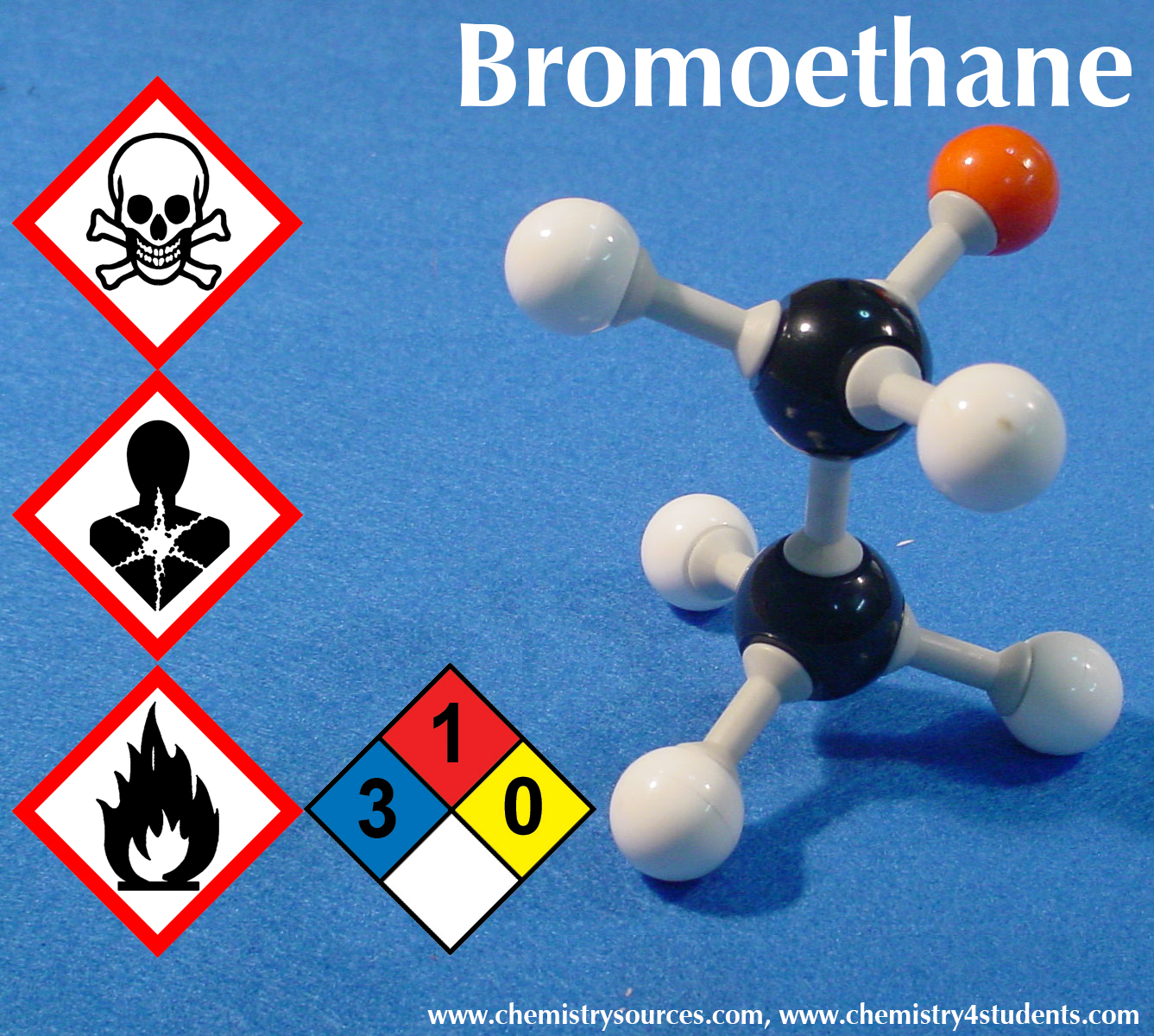 Source: chemistry4students.com
Source: chemistry4students.com
3 Calculate the percentage atom economy for the formation of D in this reaction. 3111 to 3119 K Solubility in water. Alright lets look at another SN-two reaction. Change the bond angle to see how shape affects polarity. 1 Outline the mechanism for this reaction.
1 mark 0 2. Includes kit list and safety instructions. Source of ignition - spark or high heat. Includes kit list and safety instructions. Bromine has an irritating.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title density of bromoethane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.