Density of acetylene
Home » chemistry » Density of acetyleneDensity of acetylene
Density Of Acetylene. May be fatal if swallowed and enters airways Danger Aspiration hazardH315 9043. Ethyne is known to be slightly soluble in water. Send it back for a full refund. This alkylation is coupled with catalytic turnover by the target enzyme and results in suicide inactivation of the enzymes.
 Physico Chemical Characteristics Of Acetylene Download Table From researchgate.net
Physico Chemical Characteristics Of Acetylene Download Table From researchgate.net
According to a new research report distributed by eSherpa Market Reports titled Acetylene Gas Market by Type and Distribution Channel. By heating potassium carbonate with carbon at very high temperatures he produced a residue of what is now known as potassium carbide K 2 C 2 which reacted with water to release the new gas. Typical uses oxyacetylene chemical synthesis and especially for welding and cutting. Gases - Molar Specific Heat - Molar specific heats of gases at. Ethyne is known to be slightly soluble in water. Acetylene is unstable in its purest form.
According to a new research report distributed by eSherpa Market Reports titled Acetylene Gas Market by Type and Distribution Channel.
A Selectivity as a function of acetylene conversion over Pd 10 Bi 2 O 3 TiO 2 Pd 02 Bi 2 O 3 TiO 2 and PdAg 3 Al 2 O 3. Select a material and enter the desired energies or use the default energies. Send it back for a full refund. C 6 H 6 combusts in air. A density functional theory study. The structure of the prosthetic heme adduct is -CH2CHO.
 Source: researchgate.net
Source: researchgate.net
It is shipped with dissolved acetone in its gaseous form. The PSTAR program calculates stopping power and range tables for protons in various materials. Since ethyne burns with a very hot flame one of its most notable applications is in. When comparing propane vs acetylene propane cannot be used for gas welding. The melting point of ethyne is roughly equal to -808 degrees Celsius or 1923 Kelvin.
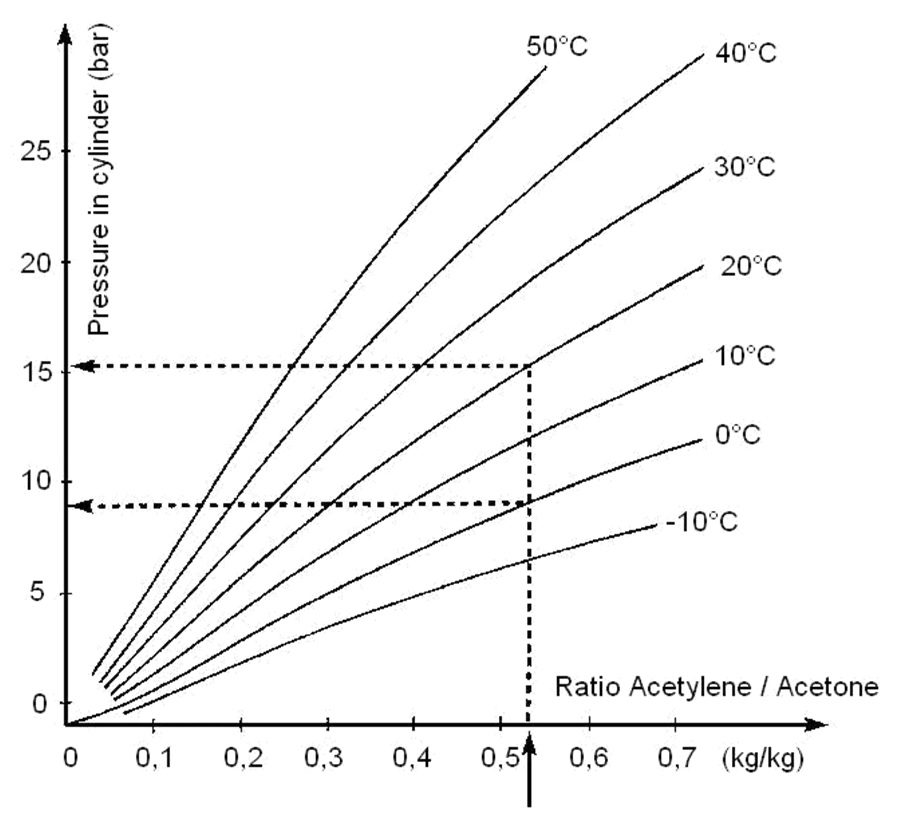 Source: gcegroup.com
Source: gcegroup.com
Acetylene was discovered in 1836 by Edmund Davy who identified it as a new carburet of hydrogen. Ethyne is known to be slightly soluble in water. C 6 H 6 combusts in air. Flammable liquid and vapor Warning Flammable liquidsH304 7652. Calcium carbide CaC 2 reacts with water to form calcium hydroxide CaOH 2 and acetylene gas C 2 H 2.
 Source: climatecolab.org
Source: climatecolab.org
Acetylene gas lamps were used to illuminate buildings as lighthouse beacons and as headlights on motor-cars and bicycles. Portable acetylene gas lamps worn on the hat or carried by hand were widely used in mining. A Selectivity as a function of acetylene conversion over Pd 10 Bi 2 O 3 TiO 2 Pd 02 Bi 2 O 3 TiO 2 and PdAg 3 Al 2 O 3. The molecular shape of the ethyne molecule is linear. This alkylation is coupled with catalytic turnover by the target enzyme and results in suicide inactivation of the enzymes.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Gases - Molar Specific Heat - Molar specific heats of gases at. C 5 H 12 O combusts in air. For example porous materials are needed for acetylene C 2 H 2 capture and separation from ethylene C 2 H 4 1317 industrial processes that are relevant for the production of polymer-grade C 2 H 2 and C 2 H 4 the most produced organic compound in the world at over 140 million metric tons in 2014. May cause respiratory irritation Warning. The reduction in bond lengths in multiple bonds points towards this statement.
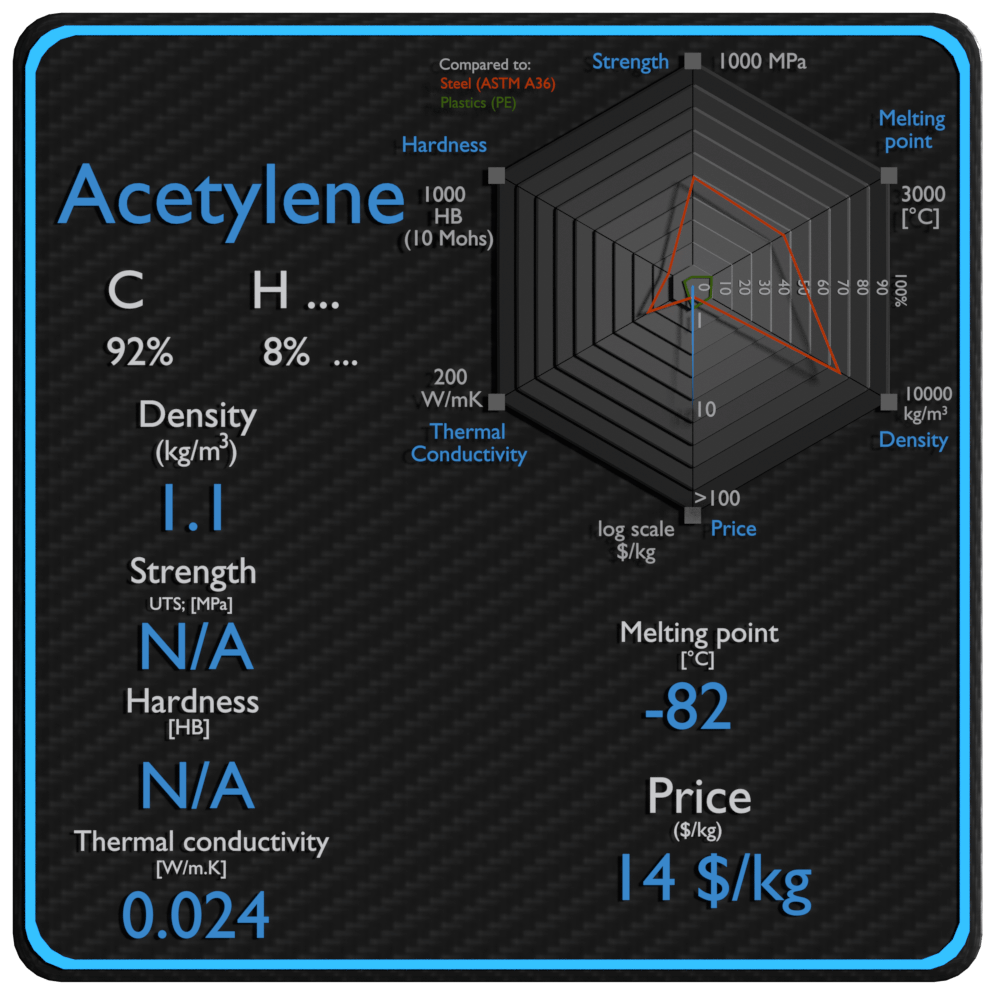 Source: material-properties.org
Source: material-properties.org
According to a new research report distributed by eSherpa Market Reports titled Acetylene Gas Market by Type and Distribution Channel. 65 10591066 2019. Acetylene Propane Torch Flame Temperature. Density Converter - Online density converter with commonly used units. Gases - Explosion and Flammability Concentration Limits - Flame and explosion limits for gases like propane methane butane acetylene and more.
 Source: numerade.com
Source: numerade.com
Not satisfied with your purchase. Specific Volume ft 3 lb m 3 kg 149 093. Portable acetylene gas lamps worn on the hat or carried by hand were widely used in mining. C 6 H 6 combusts in air. Chemical physical and thermal properties of Acetylene Ethyne - C 2 H 2.
 Source: pubs.rsc.org
Source: pubs.rsc.org
C 5 H 12 O combusts in air. Flammable liquid and vapor Warning Flammable liquidsH304 7652. Calcium carbide CaC 2 reacts with water to form calcium hydroxide CaOH 2 and acetylene gas C 2 H 2. Acetylene is odorless but commercial products normally have a marked odor. What are the Uses of Ethyne.
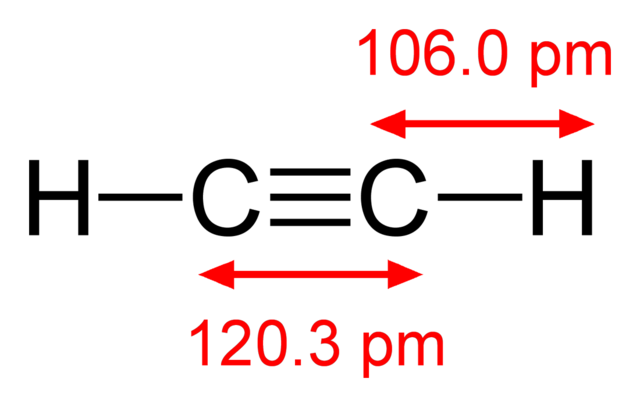 Source: en.wikipedia.org
Source: en.wikipedia.org
The MOF-74 family of compounds has a high density of open metal sites that drive high. What are the Uses of Ethyne. According to a new research report distributed by eSherpa Market Reports titled Acetylene Gas Market by Type and Distribution Channel. Gases - Molar Specific Heat - Molar specific heats of gases at. Send it back for a full refund.
 Source: researchgate.net
Source: researchgate.net
The molecular shape of the ethyne molecule is linear. High Density Abrasive Grinding Wheels Type 29 for MetalWood Grinding 78 inch Arbor Size 46 out of 5 stars 477. The PSTAR program calculates stopping power and range tables for protons in various materials. Na 2 S 2 O 3 AgBr NaBr Na 3 AgS 2 O 3 2 a. 1 offer from 1799.
 Source: numerade.com
Source: numerade.com
1 offer from 1799. Causes skin irritation Warning Skin corrosionirritationH319 9565. Carbide lamps or acetylene gas lamps are simple lamps that produce and burn acetylene C 2 H 2 which is created by the reaction of calcium carbide CaC 2 with water H 2 O. The MOF-74 family of compounds has a high density of open metal sites that drive high. Given the following reaction.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title density of acetylene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.