Density of 2 bromopropane
Home » chemistry » Density of 2 bromopropaneDensity of 2 bromopropane
Density Of 2 Bromopropane. On the other hand bottled gas can be either propane density 188 gL or butanes a mixture of butane and. Natural gas is composed chiefly of methane which has a density of about 067 gL. Note that the nominal oxidation number of the carbon bonded to the halogen changes from 1 in primary to 1. Shapes of Molecules and Electron Density of Alkanes.
 2 Bromopropane Wikipedia From en.wikipedia.org
2 Bromopropane Wikipedia From en.wikipedia.org
1014 to 1029 C. Free radical chlorination though would not be quite as selective and there would be a greater amount of the chlorination of the primary carbon than in the bromination reaction. A 1 gcm 3 b 2 gcm 3 c 3 gcm 3 d 4gcm 3 Ans 38. Iii 2-Bromopropane iv 2-Bromopropan-2-ol. Refractive index n D 1439 Thermochemistry Heat capacity C 1622 J K 1 mol 1. Xvi 2-Bromopropane to 1-bromopropane xvii Chloroethane to butane xviii Benzene to diphenyl xix tert-Butyl bromide to isobutyl bromide xx Aniline to phenylisocyanide.
A girl has an aorta radius of 13 mm.
Std enthalpy of. Alkyl groups have a tendency to push electrons away from themselves towards the double bond. Q-A 5 solution by mass of cane sugar in. Density Functional DFT Methods. On the other hand bottled gas can be either propane density 188 gL or butanes a mixture of butane and. The intermediate carbocation formed will be secondary since it is more stable than primary.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Reaction of C 6 H 5 CH 2 Br with aqueous sodium hydroxide follows a S N 1 mechanism b S N 2 mechanism c any of the above two depending upon the temperature of reaction d Saytzeff rule. Note that the nominal oxidation number of the carbon bonded to the halogen changes from 1 in primary to 1. We have previously considered electrons as being located in atomic orbitals within the subshells and shells of atoms. Alkyl groups have a tendency to push electrons away from themselves towards the double bond. 2-Bromopropane is prepared by heating isopropanol with hydrobromic acid.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Density Functional DFT Methods. The intermediate carbocation formed will be secondary since it is more stable than primary. 3745 to 3760 K log P. 40 mm Hg at 66 F. When electrons are shared between atoms to form.
 Source: gasmet.com
Source: gasmet.com
Enter the email address you signed up with and well email you a reset link. Hydrogen bonding causes the higher boiling point than expected compared to other organic molecules with similar relative molecular masses. Free radical chlorination though would not be quite as selective and there would be a greater amount of the chlorination of the primary carbon than in the bromination reaction. 3745 to 3760 K log P. Natural gas is composed chiefly of methane which has a density of about 067 gL.
 Source: en.wikipedia.org
Source: en.wikipedia.org
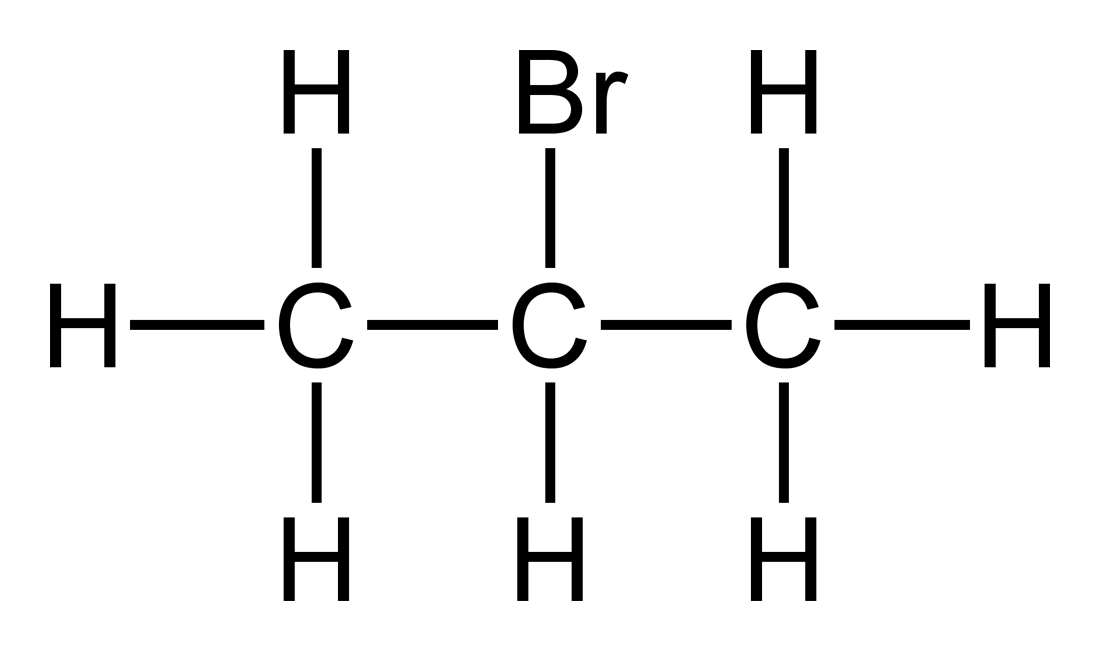
Its edge length is 050 nm. 2-Bromopropane also known as isopropyl bromide and 2-propyl bromide is the halogenated hydrocarbon with the formula CH 3 CHBrCH 3. It is a colorless liquid. 2144 to 2171 F. Anything which increases the electron density around the double bond will help this.

Classify each alkyl halide as 1 2 or 3. The more negatively charged that region becomes the more it will attract molecules like hydrogen chloride. Enter the email address you signed up with and well email you a reset link. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Xvi 2-Bromopropane to 1-bromopropane xvii Chloroethane to butane xviii Benzene to diphenyl xix tert-Butyl bromide to isobutyl bromide xx Aniline to phenylisocyanide.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Anything which increases the electron density around the double bond will help this. The addition of HBr to propene is an example of an electrophilic substitution reaction. Reaction of C 6 H 5 CH 2 Br with aqueous sodium hydroxide follows a S N 1 mechanism b S N 2 mechanism c any of the above two depending upon the temperature of reaction d Saytzeff rule. 12676 g mL 1. 1-Chloropentane and 2-Chloropentane have the same molecular formula of C5H11Cl.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Xvi 2-Bromopropane to 1-bromopropane xvii Chloroethane to butane xviii Benzene to diphenyl xix tert-Butyl bromide to isobutyl bromide xx Aniline to phenylisocyanide. Propene reacts with HBr to form 2-bromopropane. A It reacts with metallic Na to give ethane. ILO International Chemical Safety Cards ICSC 159. Calculate the quantity of CO 2 in 500 mL of soda water when packed under 25 atm CO 2 pressure at 298 K.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
B It gives nitroethane on heating with aqueous solution of AgNO2 c It gives C2H5OH on boiling with alcoholic potash. The more negatively charged that region becomes the more it will attract molecules like hydrogen chloride. 60 CH 3 3 CCl. Physics QA Library Human blood flows from the aorta to the capillaries. Which of the following is a correct statement for C2H5Br.

Xvi 2-Bromopropane to 1-bromopropane xvii Chloroethane to butane xviii Benzene to diphenyl xix tert-Butyl bromide to isobutyl bromide xx Aniline to phenylisocyanide. The more alkyl groups you have the more negative the area around the double bonds becomes. 40 mm Hg at 66 F. Which one of the following are correctly arranged on the basis of the property indicated. It is a colorless liquid.
 Source: molinstincts.com
Source: molinstincts.com
Calculate the density if molar mass of NaCl 585 gmol. C2H5OH 3O2 2 O2 3H2O Substitution forming halogenoalkane nucleophilic substitution. 2-Bromopropane is prepared by heating isopropanol with hydrobromic acid. 12676 g mL 1. Being an acid the hydrogen bromide provides an electrophile H.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title density of 2 bromopropane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.