Density of 1 chlorobutane
Home » chemistry » Density of 1 chlorobutaneDensity of 1 chlorobutane
Density Of 1 Chlorobutane. Butyl chloride NF HSDB 4167. 1-Chlorobutane 995 anhydrous AcroSealR EINECS 203-696-6. I Butane 1-Chlorobutane 1-Bromobutane 1-Iodobutane Explanation. C5h11cl isomers - egzotyka-lastminutepl.

C5h11cl isomers - egzotyka-lastminutepl. Density of Gas M. 1-Chlorobutane 998 for HPLC. It readily communicates whether a water-insoluble solvent will float SG 10 or sink SG 10 when mixed with water. Chlorobutane 1-Chlorure de butyle French CAS-109-69-3. Your table should include tert-pentyl alcohol volume mass mmoles density equivalents and boiling point and concentrated HCl volume mmoles equivalents Volume mL Mass g mmoles Density Equiv Boiling PointProperties tert-pentyl alcohol 60 48 55 0805.
A but-2-ene b 1- bromobutane c 2 -bromobutane d 1 butanol.
Coefficient of cubical expansion. Nucleophiles seek centers of low electron density. 0586 calgdeg C at 25 C. Ii 2-Bromobutane is chiral therefore optically active whereas 1 -chlorobutane is not chiral therefore optically inactive. 2 Li C 4 H 9 X C 4 H 9 Li LiX X Cl Br If the lithium used for this reaction contains 13 sodium the reaction proceeds more quickly than if pure lithium is used. Iii It is due to I effect of halogens it deactivates benzene ring towards electrophilic substitution reactions.

Coefficient of cubical expansion. Which of the following statements related to SN1 reactions is not true. ここで気体の密度を0とすると次のように変形できる γ PV 4 lnγ 4lnP 4lnV もしParachorが温度依存性の無い物性値であるなら式の温度微分は次のようになる d ln γdT -40 d lnVdT. This tutorial leads you through the process of building. It readily communicates whether a water-insoluble solvent will float SG 10 or sink SG 10 when mixed with water.
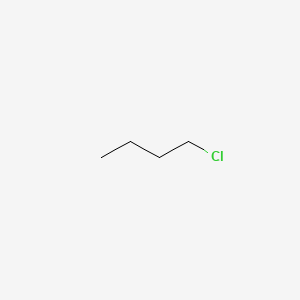
Thus the boiling point of isopropyl alcohol is lower than that of 1-chloropropane. For the same alkyl group the boiling points of alkyl halides decrease in the order. Iv Butane 1-Chlorobutane 1-Iodobutane 1-BromobutaneSolution. Butyl chloride NF HSDB 4167. The I-MASK Prophylaxis and Early Outpatient Treatment Protocol for COVID-19 and the MATH Hospital Treatment Protocol for COVID-19 are physiologic-based combination treatment regimen created by the FLCCC Alliance a group of leaders in critical care medicine.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
When compound A is react with HBr it form compound B the compound B is. Ii 2-Bromobutane is chiral therefore optically active whereas 1 -chlorobutane is not chiral therefore optically inactive. Also contains all the key points discussed in this post MOC_Boiling_Point_Handout PDF Figuring out the order of. Butyl chloride NF HSDB 4167. Wir beraten Sie gerne.

Pentane 0626 Petroleum ether 0656 Hexane 0659 Heptane 0684 Diethyl amine 0707. Which is the correct increasing order of boiling points of the following compounds. This is because with the increase in size and mass of halogen atom the magnitude of van der Waal forces increases. Iv Butane 1-Chlorobutane 1-Iodobutane 1-BromobutaneSolution. This tutorial leads you through the process of building.
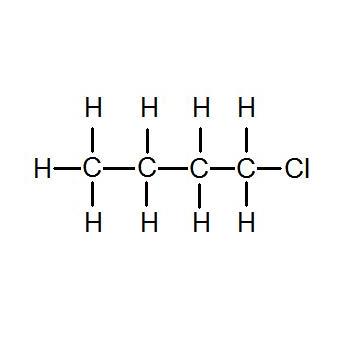
0586 calgdeg C at 25 C. Further the boiling point decreases with an increase in branching in the chain. 956X10-7 at 0-94 C. The charged carbon atom of a carbocation has a complete octet of valence shell electrons. These 3 commercially available compounds have molecular formula C4H9F.

For the same alkyl group the boiling points of alkyl halides decrease in the order. X axis coincident with C 2 axis D 4h. ここで気体の密度を0とすると次のように変形できる γ PV 4 lnγ 4lnP 4lnV もしParachorが温度依存性の無い物性値であるなら式の温度微分は次のようになる d ln γdT -40 d lnVdT. Which is the correct increasing order of boiling points of the following compounds. Ii 2-Bromobutane is chiral therefore optically active whereas 1 -chlorobutane is not chiral therefore optically inactive.
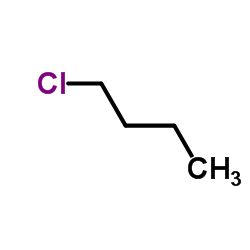 Source: chemsrc.com
Source: chemsrc.com
Haloalkanes and Haloarenes Previous Year Question 11. Isomers of c4h9f email protected 371 67881775. Isomers of c5h11cl. With 10 examples of solved problems. View Answer Why is 1-chlorobutane gives precipitate with sodium iodide in acetone reaction while 1-iodopropane does not even though they are both primarily alkyl.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Boiling point increases with increase in molecular mass of halogen atom for the similar type of alkyl halide. 2 Li C 4 H 9 X C 4 H 9 Li LiX X Cl Br If the lithium used for this reaction contains 13 sodium the reaction proceeds more quickly than if pure lithium is used. Which of the following statements related to SN1 reactions is not true. A The higher the surface area the higher will be the intermolecular forces of attraction and thus boiling point too. X axis coincident with C 2 axis D 4h.
 Source: carbosynth.com
Source: carbosynth.com
1-Chlorobutane 998 for HPLC. Thus the boiling point of 1-chlorobutane is higher than that of isopropyl chloride and 1-chloropropane. Which of the following statements related to SN1 reactions is not true. From the straight-chain alkane we get 1-Chlorobutane and 2-Chlorobutane From the branched-chain alkane we get 1-Chloro-2-methylpropane and 2-Chloro-2 Another example of constitutional isomers is 12-dibromocyclohexane and 14-dibromocyclohexane. Guidechem provides Cyclopentyl chloride chemical database query including CAS registy number 930-28-9 Cyclopentyl chloride MSDS Material Safety Data Sheet nature English name manufacturer functionuse molecular weight density boiling point melting point structural formula etc.
 Source: molinstincts.com
Source: molinstincts.com
Isopropyl chloride 1. Which is the correct increasing order of boiling points of the following compounds. Density is a chemical property. Density of Gas M. Chlorobutane 1-Chlorure de butyle French CAS-109-69-3.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title density of 1 chlorobutane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.