Copper ii sulfate pentahydrate density
Home » chemistry » Copper ii sulfate pentahydrate densityCopper ii sulfate pentahydrate density
Copper Ii Sulfate Pentahydrate Density. What volume of 0407 M KOH is just sufficient to react completely with 316 grams of copper II sulfate pentahydrate according to the equation below. Good set forth several criteria for such buffers. 1 Structures Expand this section. Cupric sulfate pentahydrate Sulfuric acid copper2 salt pentahydrate.
 Material Safety Data Sheet Copper Sulfate Solution From studylib.net
Material Safety Data Sheet Copper Sulfate Solution From studylib.net
Hold eyelids apart and flush eyes with plenty of water for at least 15 minutes. CopperII sulfate CuSO 4 is white in is anhyrous form and deep blue when it is complexed with water in the pentahydrate form CuSO 4 5H 2 O. First Aid Measures Description of First Aid Measures. The biuret reagent contains sodium hydroxide copper II sulfate and potassium sodium tartrate. 4 Spectral Information Expand this section. Weigh out approximately 10 g of copper II sulfate pentahydrate.
Academiaedu is a platform for academics to share research papers.
It has a role as a sensitiser a fertilizer and an emetic. The existing form is CrVII and the form of copper ions under corrosive conditions is CuII so when the fiber oozes different metal ions it has better permeability to copper ions. Specific gravity density 67. H410 7732-18-5 ZC0110000 Water 750 -900 231-791-2 NA No data available. What is the freezing point of a solution prepared by adding 2390 g of copperII sulfate pentahydrate to 400 liters of water. The most common oxidation states of iron are Cu the copperI or cuprous ion and Cu 2 the copperII or cupric ion.

View Answer An 875-g sample of iron ore mixture is transformed to a solution of ironII sulfate and this solution is titrated with 0. CopperII sulfate pentahydrate 99 for analysis. 500 mole 4 P 5 O 2 à P 4O 10 14. CopperII sulfate pentahydrate 98 ACS reagent. Weigh out approximately 10 g of copper II sulfate pentahydrate.
 Source: studylib.net
Source: studylib.net
The biuret reagent contains sodium hydroxide copper II sulfate and potassium sodium tartrate. Is iron sulphide compounds. The hydrate dissolves as the KOH is added to f. The existing form is CrVII and the form of copper ions under corrosive conditions is CuII so when the fiber oozes different metal ions it has better permeability to copper ions. H319 Aquatic A 1.
 Source: chemistry-reference.com
Source: chemistry-reference.com
The table below gives calculated values of K. The hydrate dissolves as the KOH is added to f. H315 Eye Damage 2. 1 Structures Expand this section. Note the use of a van t Hoff factor.
 Source: studylib.net
Source: studylib.net
H315 Eye Damage 2. 3CuOH 2 CuSO 4. M m A n s mM n aq nA m-aq. 4 Spectral Information Expand this section. This dark blue to purple solid is a salt of the metal complex CuNH 3 4 H 2 O 2It is closely related to Schweizers reagent which is used for the production of cellulose fibers in the production of rayon.
 Source: yumpu.com
Source: yumpu.com
Light blue green fine powder. For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation. The rate of the reaction can be affected by the type of reaction as well as the concentration. CuSO 4 5H 2 O. Copper sulfate CuSO4 pentahydrate.

The leader in Cannabis Science Supplies Equipment and Information. H410 7732-18-5 ZC0110000 Water 750 -900 231-791-2 NA No data available. 5 Related Records Expand this. Note the use of a van t Hoff factor. 1 Structures Expand this section.
 Source: studylib.net
Source: studylib.net
CopperII sulfate pentahydrate 98 ACS reagent. Stir thoroughly to make certain that all the copper salt has dissolved before proceeding. CopperII sulfate is a metal sulfate compound having copper2 as the metal ion. Weigh out approximately 10 g of copper II sulfate pentahydrate. 5 g ore sample that contains 1.
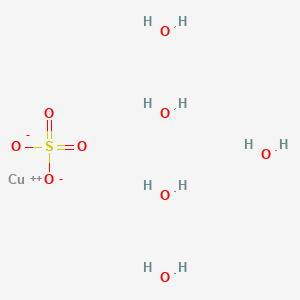
5 g ore sample that contains 1. View Answer An 875-g sample of iron ore mixture is transformed to a solution of ironII sulfate and this solution is titrated with 0. H410 7732-18-5 ZC0110000 Water 750 -900 231-791-2 NA No data available. Blue crystals granules or powder. H400 Aquatic C 1.
 Source: studylib.net
Source: studylib.net
Is iron sulphide compounds. CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula Cu SO 4 H 2 O x where x can range from 0 to 5The pentahydrate x 5 is the most common form. The rate of the reaction can be affected by the type of reaction as well as the concentration. CopperII sulfate is a metal sulfate compound having copper2 as the metal ion. The complex ion is the anion so we have to add the suffix ate in the name of the metal.

First Aid Measures Description of First Aid Measures. The molar mass of copperII sulfate pentahydrate is 24968 gmol. Cupric sulfate pentahydrate Sulfuric acid copper2 salt pentahydrate. 2 Names and Identifiers Expand this section. The freezing point depression of water is 186 Cm.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title copper ii sulfate pentahydrate density by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.