2 bromopropane density
Home » chemistry » 2 bromopropane density2 bromopropane density
2 Bromopropane Density. The concurrent substitution and elimination reactions of a halogenoalkane eg 2-bromopropane with potassium hydroxide. Addition of HBr to propene yields 2-bromopropane while in the presence of benzoyl peroxide the same reaction yields 1-bromopropane. When electrons are shared between atoms to form. 60 CH 3 3 CCl.
 2 Bromopropane 99 Thermo Scientific Alkyl Halides Organohalogen Compounds Fisher Scientific From fishersci.co.uk
2 Bromopropane 99 Thermo Scientific Alkyl Halides Organohalogen Compounds Fisher Scientific From fishersci.co.uk
Addition of HBr to propene yields 2-bromopropane while in the presence of benzoyl peroxide the same reaction yields 1-bromopropane. Students should be able to. 25 g L 1 at 20 C Solubility in ethanol. Note that the nominal oxidation number of the carbon bonded to the halogen changes from 1 in primary to 1. Being an acid the hydrogen bromide provides an electrophile H. If propane CH 3 CH 2 CH 3 for example was the substrate 2-bromopropane would be the dominant product and there would be only a small amount of 1-bromopropane.
Density Functional DFT Methods.
Being an acid the hydrogen bromide provides an electrophile H. Outline the mechanisms of these reactions. C 2-Bromopropane d 2-Bromopropan-2-ol Solution. Its edge length is 050 nm. Option i is the answer. When a natural-gas leak is detected and shut off in a room the gas can be removed by opening an upper window.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1607 K Boiling point. A Both A and R are true and R is the correct explanation of A b Both A and R are true but R is. Natural gas is composed chiefly of methane which has a density of about 067 gL. The objects which are nonsuperimposable on their mirror images are called enantiomers. This is because each carbon atom forms four bonding pairs leaving no lone pairs.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
A 1 gcm 3 b 2 gcm 3 c 3 gcm 3 d 4gcm 3 Ans 38. Explain and give mechanism. The concurrent substitution and elimination reactions of a halogenoalkane eg 2-bromopropane with potassium hydroxide. Propene reacts with HBr to form 2-bromopropane. Reaction of C6H5CH2Br with aqueous sodium hydroxide follows _____.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Relative vapor density air 1. This electrophile attacks the propene double bond to form. Last updated on. A unit cell of NaCl has 4 formula units. Outline the mechanisms of these reactions.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Reaction of C6H5CH2Br with aqueous sodium hydroxide follows _____. Natural gas is composed chiefly of methane which has a density of about 067 gL. National Toxicology Program Chemical. The density of air is about 129 gL. Option i is the answer.
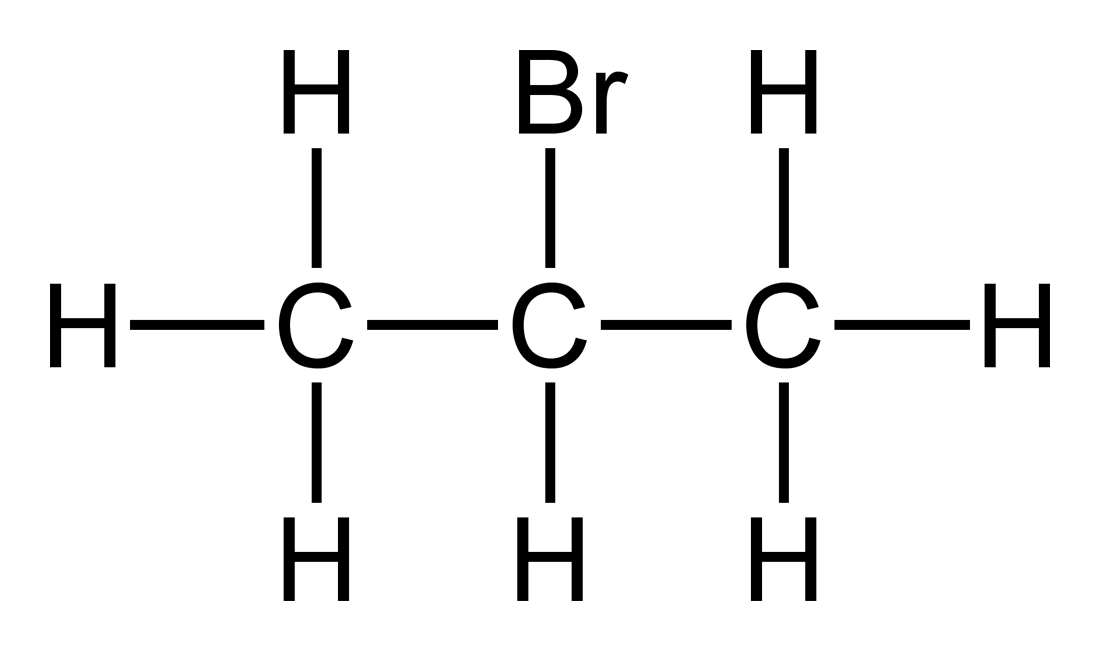 Source: en.wikipedia.org
Source: en.wikipedia.org
Density Functional DFT Methods. When a natural-gas leak is detected and shut off in a room the gas can be removed by opening an upper window. 50 mm Hg at 77 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. This is because each carbon atom forms four bonding pairs leaving no lone pairs. 1014 to 1029 C.
 Source: sielc.com
Source: sielc.com
Addition of HBr to propene yields 2-bromopropane while in the presence of benzoyl peroxide the same reaction yields 1-bromopropane. Write balanced equations for the complete combustion of the following hydrocarbons. When electrons are shared between atoms to form. Free radical chlorination though would not be quite as selective and there would be a greater amount of the chlorination of the primary carbon than in the bromination reaction. The objects which are nonsuperimposable on their mirror images are called enantiomers.
 Source: molinstincts.com
Source: molinstincts.com
Refractive index n D 1. 3745 to 3760 K log P. A I 2 Br 2 F 2 Cl 2 increasing bond. 1584 to 1602 F. The phenoxide ion is more resonance stabilised than alkoxide ion.

53 kPa Henrys law constant k H 140 nmol Pa kg 1. Explain the role of the reagent as both nucleophile and base. The concurrent substitution and elimination reactions of a halogenoalkane eg 2-bromopropane with potassium hydroxide. When a natural-gas leak is detected and shut off in a room the gas can be removed by opening an upper window. Std enthalpy of.
 Source: tcichemicals.com
Source: tcichemicals.com
Note that the nominal oxidation number of the carbon bonded to the halogen changes from 1 in primary to 1. This is because each carbon atom forms four bonding pairs leaving no lone pairs. 2144 to 2171 F. Q- Henrys law constant for CO 2 in water is 167 x 10 8 Pa at 298 K. Ozone formed naturally in the upper atmosphere is beneficial.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Refractive index n D 1439 Thermochemistry Heat capacity C 1622 J K 1 mol 1. When a natural-gas leak is detected and shut off in a room the gas can be removed by opening an upper window. Last updated on. Option i is the answer. Refractive index n D 1439 Thermochemistry Heat capacity C 1622 J K 1 mol 1.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title 2 bromopropane density by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.