1 chlorobutane density
Home » chemistry » 1 chlorobutane density1 chlorobutane density
1 Chlorobutane Density. This tutorial leads you through the process of building. C5h11cl isomers - egzotyka-lastminutepl. A Number of moles b Mass c Volume d Density 6. ここで気体の密度を0とすると次のように変形できる γ PV 4 lnγ 4lnP 4lnV もしParachorが温度依存性の無い物性値であるなら式の温度微分は次のようになる d ln γdT -40 d lnVdT.
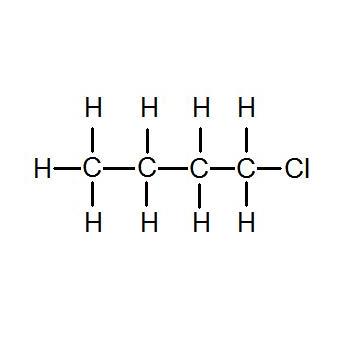
Haloalkanes and Haloarenes Previous Year Question 11. Density is a chemical property. A Number of moles b Mass c Volume d Density 6. C5h11cl isomers - cp-2077pl. The I-MASK Prophylaxis and Early Outpatient Treatment Protocol for COVID-19 and the MATH Hospital Treatment Protocol for COVID-19 are physiologic-based combination treatment regimen created by the FLCCC Alliance a group of leaders in critical care medicine. Q-An antifreeze solution is prepared from 2226 g of ethylene glycol C 2 H 6 O 2 and 200 g of water.
Q-An antifreeze solution is prepared from 2226 g of ethylene glycol C 2 H 6 O 2 and 200 g of water.
Academiaedu is a platform for academics to share research papers. This is because with the increase in size and mass of halogen atom the magnitude of van der Waal forces increases. The relative lowering of vapour pressure of a solvent by the addition of a solute is. Q-Identify allylic alcohols in the above examples. Haloalkanes and Haloarenes Previous Year Question 11. Tk and Then put a Br atom in every possible location on each of the carbon skeletons.

Density of Gas M. Iv Butane 1-Chlorobutane 1-Iodobutane 1-BromobutaneSolution. N-Butyllithium C 4 H 9 Li abbreviated n-BuLi is an organolithium reagentIt is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene SBSAlso it is broadly employed as a strong base in the synthesis of organic compounds as in the pharmaceutical industry. For the same alkyl group the boiling points of alkyl halides decrease in the order. Which is the correct increasing order of boiling points of the following compounds.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Noyes Data Corporation Park Ridge NJ 1991 p. A but-2-ene b 1- bromobutane c 2 -bromobutane d 1 butanol. A Number of moles b Mass c Volume d Density 6. Coefficient of cubical expansion. ここで気体の密度を0とすると次のように変形できる γ PV 4 lnγ 4lnP 4lnV もしParachorが温度依存性の無い物性値であるなら式の温度微分は次のようになる d ln γdT -40 d lnVdT.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
X axis coincident with C 2 axis D 4h. Butyl chloride NF HSDB 4167. For the same alkyl group the boiling points of alkyl halides decrease in the order. This tutorial leads you through the process of building. 2X10-3 mhocm at 25 C.

Your table should include tert-pentyl alcohol volume mass mmoles density equivalents and boiling point and concentrated HCl volume mmoles equivalents Volume mL Mass g mmoles Density Equiv Boiling PointProperties tert-pentyl alcohol 60 48 55 0805. Butyllithium is commercially available as solutions 15 25 1. Thus the boiling point of isopropyl alcohol is lower than that of 1-chloropropane. ここで気体の密度を0とすると次のように変形できる γ PV 4 lnγ 4lnP 4lnV もしParachorが温度依存性の無い物性値であるなら式の温度微分は次のようになる d ln γdT -40 d lnVdT. 2X10-3 mhocm at 25 C.
 Source: molinstincts.com
Source: molinstincts.com
View Answer Why is 1-chlorobutane gives precipitate with sodium iodide in acetone reaction while 1-iodopropane does not even though they are both primarily alkyl. For the same alkyl group the boiling points of alkyl halides decrease in the order. Density of Gas M. View Answer Why is 1-chlorobutane gives precipitate with sodium iodide in acetone reaction while 1-iodopropane does not even though they are both primarily alkyl. 1-Chlorobutane 995 anhydrous AcroSealR EINECS 203-696-6.
 Source: chemsrc.com
Source: chemsrc.com
Further the boiling point decreases with an increase in branching in the chain. Which is the correct increasing order of boiling points of the following compounds. Q-Identify allylic alcohols in the above examples. Thus the boiling point of isopropyl alcohol is lower than that of 1-chloropropane. 2X10-3 mhocm at 25 C.
 Source: carbosynth.com
Source: carbosynth.com
If the density of solution is 12 g mL-1. The solubility of a gas varies directly with pressure of the gas is based upon. Q-Identify allylic alcohols in the above examples. Industrial Solvents Handbook 4 th ed. Haloalkanes and Haloarenes Previous Year Question 11.

A Number of moles b Mass c Volume d Density 6. An example of intensive property is. N-Butyllithium C 4 H 9 Li abbreviated n-BuLi is an organolithium reagentIt is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene SBSAlso it is broadly employed as a strong base in the synthesis of organic compounds as in the pharmaceutical industry. If the density of solution is 12 g mL-1. 67 lb at 20 C.
For the same alkyl group the boiling points of alkyl halides decrease in the order. 1-Chlorobutane 995 anhydrous AcroSealR EINECS 203-696-6. Hazardous Substances Data Bank HSDB Heat capacity liquid 25 C 141 J. I Butane 1-Chlorobutane 1-Bromobutane 1-Iodobutane Explanation. As such specific gravity is a unitless value.

Option i is the answer. 956X10-7 at 0-94 C. The relative lowering of vapour pressure of a solvent by the addition of a solute is. Hazardous Substances Data Bank HSDB Heat capacity liquid 25 C 141 J. A Raoults Law b Henrys Law c Nernsts Distribution Law d None of these 7.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title 1 chlorobutane density by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.